A study of 76,000 Swedish adults found that strict adherence to the Nordic diet is linked to a 23 percent drop in premature death plus lower cancer and heart disease mortality.



A low dose of aspirin each day may significantly reduce the chances of colon and rectal cancer returning in certain cases, a new clinical trial has found.
Led by researchers from the Karolinska Institute and Karolinska University Hospital in Sweden, the study involved 626 people with stages 1 to 3 colon or rectal cancer, and specific genetic mutations in the cancer tumors.
Previous studies have suggested that cancers with these mutations – specifically in the PIK3 signaling pathway – could be targeted by aspirin, but this is the first time the hypothesis has been tested in a randomized clinical trial.
Genetic engineering and human enhancement are no longer science fiction — they’re here right now. In this episode of Longevity Science News, we explore the rise of gene therapy, anti-aging biotechnology, and the first wave of GMO Humans using real genetic enhancements to increase muscle, extend telomeres, boost IQ, and slow biological aging.
If you’re interested in longevity, life extension, biohacking, genetic modification, or cutting-edge anti-aging research, this video breaks down everything you need to know about the future of human evolution — and the people already jumping in.
HUME BODY ANALYZER:
Use Code: LONGEVITY for up to 50% OFF
https://humehealth.com//discount/LONG… FEATURED: BioViva Keynote by Liz Parrish Watch the full keynote here: • The First Person to Take Gene Therapy for… This talk covers viral vectors, telomere extension, muscle-growth gene therapies, cognitive enhancement, dementia treatment, and the global expansion of experimental genetic clinics. Chapters: 00:00 – Cold Open — FDA Gene Cures 00:35 – Liz Parrish & BioViva 01:35 – Sebastian A. Brunemeier 02:48 – HUME Body Pod 03:55 – Currently Available Genetic Cures 04:48 – How To Get Access 08:00 – Safety & Pricing 08:22 – Right to Try Debate 09:20 – Follistatin Results 10:50 – Telomere Extension 11:50 – Klotho & IQ Boost 13:26 – IQ & Society 14:35 – Dementia Gene Therapy 15:40 – Custom Therapies 16:20 – Conclusion • FDA-approved genetic cures • BioViva’s gene enhancement results • Follistatin gene therapy for muscle growth • Telomerase (TERT) for biological age reversal • Klotho gene therapy for cognitive enhancement • Dementia gene therapy case studies • Medical tourism for experimental gene treatments • How to access unapproved gene therapies • AI’s role in designing next-gen genetic interventions • Personalized & bespoke gene therapies • Ethical questions about enhancing IQ, strength, and lifespan • The future of human evolution & GMO humans 👤 EXPERTS & SOURCES FEATURED Liz Parrish — BioViva Sciences LinkedIn:
/ lizlparrish Sebastian Brunemeier — Cambrian Bio / Long Game Ventures LinkedIn:
/ sebastianlongbio Long Game Ventures:
/ longgame-vc Wired Magazine — Medical Tourism & Gene Therapy Pricing https://www.wired.com/story/bioviva-g… Extended Interview: Montana Senator Ken Bogner
• Ken Bogner Full Interview 🔗 FULL INTERVIEWS & BONUS CONTENT Get extended conversations, deep dives, and behind-the-scenes research on Patreon: 👉
/ u29506604 💬 JOIN THE DISCUSSION Would you use gene therapy to slow aging? Would you enhance your muscle, intelligence, or longevity? Do you think we should expand access to experimental anti-aging treatments? Let me know in the comments. 🧪 Longevity Science News PRODUCTION CREDITS ⎺⎺⎺⎺⎺⎺⎺⎺⎺⎺⎺⎺⎺⎺⎺⎺⎺⎺⎺⎺ Executive Producer – Keith Comito @Retromancers Host, Producer, Writer – @emmettshort
🔬 FEATURED: BioViva Keynote by Liz Parrish.
Watch the full keynote here:
• The First Person to Take Gene Therapy for…
This talk covers viral vectors, telomere extension, muscle-growth gene therapies, cognitive enhancement, dementia treatment, and the global expansion of experimental genetic clinics.
Chapters:
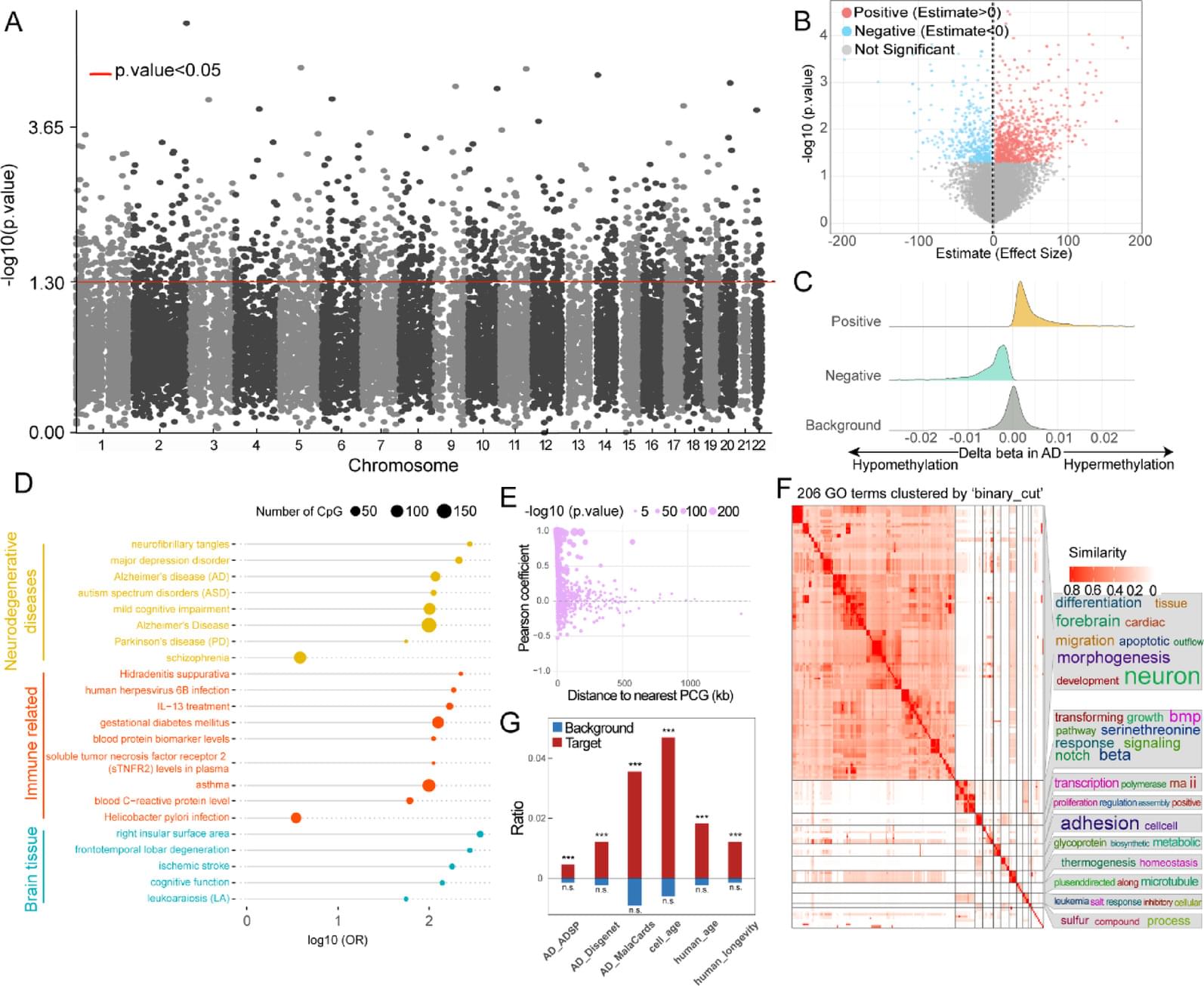
DNA methylation has shown great potential in Alzheimer’s disease (AD) blood diagnosis. However, the ability of long non-coding RNAs (lncRNAs), which can be modified by DNA methylation, to serve as noninvasive biomarkers for AD diagnosis remains unclear.
We performed logistic regression analysis of DNA methylation data from the blood of patients with AD compared and normal controls to identify epigenetically regulated (ER) lncRNAs. Through five machine learning algorithms, we prioritized ER lncRNAs associated with AD diagnosis. An AD blood diagnosis model was constructed based on lncRNA methylation in Australian Imaging, Biomarkers, and Lifestyle (AIBL) subject and verified in two large blood-based studies, the European collaboration for the discovery of novel biomarkers for Alzheimer’s disease (AddNeuroMed) and the Alzheimer’s Disease Neuroimaging Initiative (ADNI). In addition, the potential biological functions and clinical associations of lncRNAs were explored, and their neuropathological roles in AD brain tissue were estimated via cross-tissue analysis.
We characterized the ER lncRNA landscape in AD blood, which is strongly related to AD occurrence and process. Fifteen ER lncRNAs were prioritized to construct an AD blood diagnostic and nomogram model. The receiver operating characteristic (ROC) curve and the decision and calibration curves show that the model has good prediction performance. We found that the targets and lncRNAs were correlated with AD clinical features. Moreover, cross-tissue analysis revealed that the lncRNA ENSG0000029584 plays both diagnostic and neuropathological roles in AD.
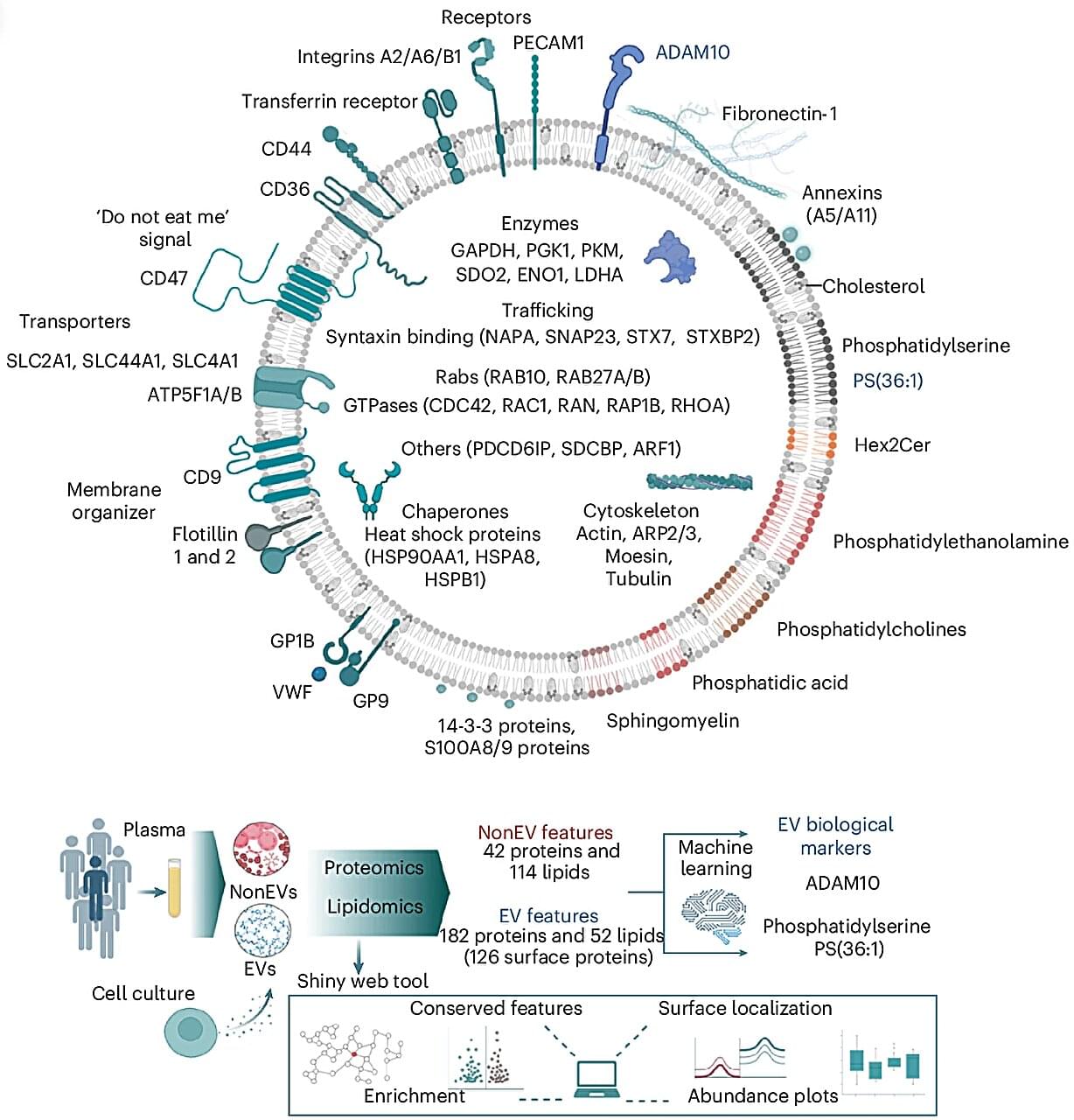
Every second, trillions of tiny parcels travel through your bloodstream—carrying vital information between your body’s cells. Now, scientists at the Baker Heart and Diabetes Institute have opened this molecular mail for the first time, revealing its contents in astonishing detail.
In research published in Nature Cell Biology, Professor David W. Greening and Dr. Alin Rai have mapped the complete molecular blueprint of extracellular vesicles (EVs)—nanosized particles in blood that act as the body’s secret messengers.
For decades, researchers have known that EVs exist, ferrying proteins, fats, and genetic material that mirror the health of their cells of origin. But because blood is a complex mixture—packed with cholesterol, antibodies, and millions of other particles—isolating EVs has long been one of science’s toughest challenges.

The International Agency for Research on Cancer (IARC) of the World Health Organization has classified hepatitis D virus (HDV) as carcinogenic, citing sufficient evidence and placing it alongside hepatitis B virus (HBV) and hepatitis C virus (HCV) as a cause of hepatocellular carcinoma (HCC).
WHO’s classification of hepatitis D virus as carcinogenic raises urgent questions for vaccination, screening, and treatment strategies worldwide.
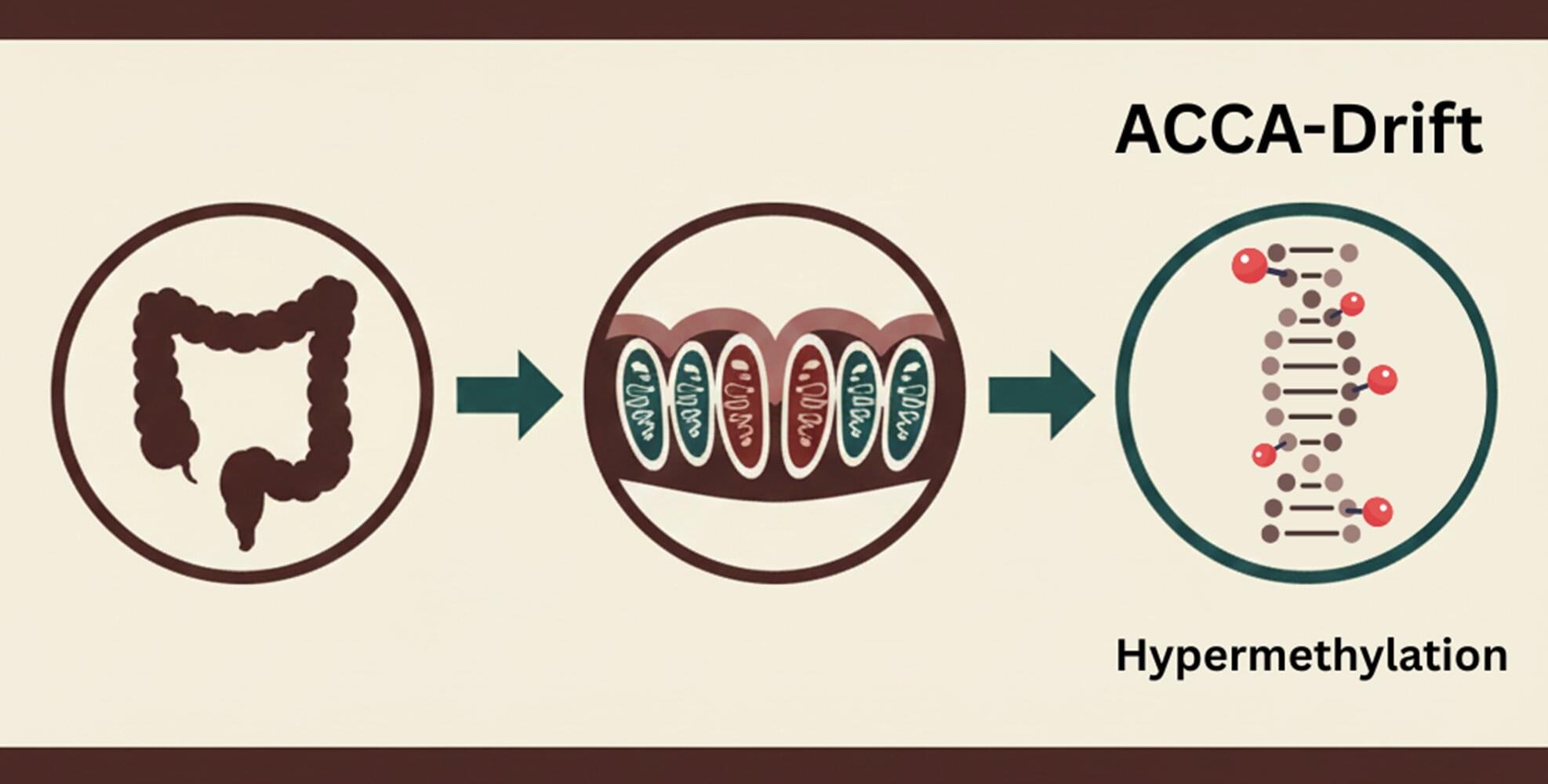
The human gut renews itself faster than any other tissue: every few days, new cells are created from specialized stem cells. However, as we get older, epigenetic changes build up in these stem cells. These are chemical markers on the DNA that act like switches, determining which genes remain active.
The study, recently published in Nature Aging, was conducted by an international team led by Prof. Francesco Neri from the University of Turin, Italy, and shows that changes in the gut do not occur randomly. Rather, a specific pattern develops over the course of aging, which the researchers refer to as ACCA (Aging-and Colon Cancer-Associated) drift. “We observe an epigenetic pattern that becomes increasingly apparent with age,” explains Prof. Neri, former group leader at the Leibniz Institute on Aging—Fritz Lipmann Institute in Jena.
Genes that maintain the balance in healthy tissue are particularly affected, including those that control the renewal of the intestinal epithelium via the Wnt signaling pathway. The changes described as “drifting” can be detected not only in the aging gut, but also in almost all colon cancer samples examined. This suggests that the aging of stem cells creates an environment that promotes the development of cancer.
Deep within your bone marrow, a specialized set of stem cells is busy pumping out new blood cells to sustain your body. As we age, these hematopoietic stem cells (or HSCs) become less productive, affecting our immune system and increasing our risk of conditions like anemia and cancer.
Now, scientists have found a way to rewind the clock in aging HSCs, which could potentially help to treat age-related blood and immune deficiencies.
Like most of our cells, HSCs contain tiny compartments known as lysosomes. These are the cells’ recycling centers, where complex molecules like proteins and lipids are sent to be broken down into smaller, reusable parts.
