Discover the power of gene editing, including the widely used CRISPR-Cas9 technology, and explore major publicly traded CRISPR companies.

One of ray kurzweils wonder machines are already here self assembled nanobots called foglet machines. This could allow for future avatars that people could pilot for daily life.
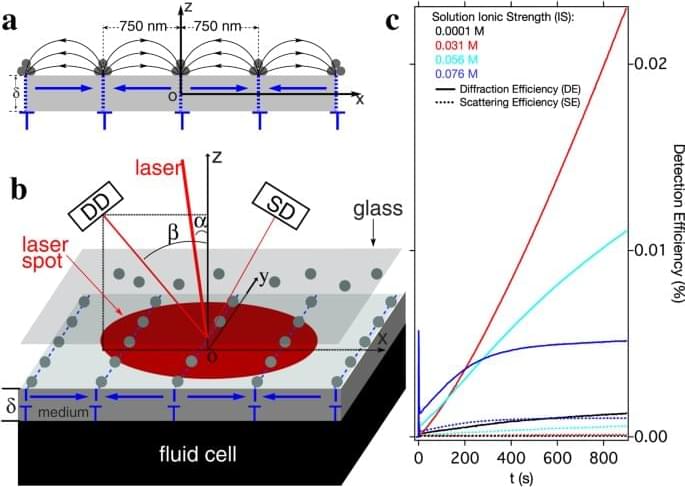
Colloidal magnetic nanoparticles are candidates for application in biology, medicine and nanomanufac-turing. Understanding how these particles interact collectively in fluids, especially how they assemble and aggregate under external magnetic fields, is critical for high quality, safe, and reliable deployment of these particles. Here, by applying magnetic forces that vary strongly over the same length scale as the colloidal stabilizing force and then varying this colloidal repulsion, we can trigger self-assembly of these nanoparticles into parallel line patterns on the surface of a disk drive medium. Localized within nanometers of the medium surface, this effect is strongly dependent on the ionic properties of the colloidal fluid but at a level too small to cause bulk colloidal aggregation.
5th BigBrain Workshop 2021
22 September 2021 — Applications.
Chair: Kathleen Rockland.
The Unique Cytoarchitecture and Wiring of The Default Mode Network.
Casey Paquola.
Background. Complex behaviours benefit from parallel distributed processing in multiple brain networks. The roles of certain networks are well-defined, while others remain elusive. Arguably, none are so elusive as the default mode network (DMN); a distributed set of brain regions that decrease in activity during many externally oriented tasks. Revealing the cytoarchitectural composition and connectional layout of the DMN is crucial to defining its role in complex behaviours.
Method. We examined the cytoarchitectural composition of the DMN using an established cortical type atlas (García-Cabezas et al., 2020; Von Economo and Koskinas, 1925) and by applying non-linear dimensionality reduction to BigBrain-derived staining intensity profiles (Paquola et al., 2019). Next, we used magnetic resonance imaging (MRI) to explicate structural wiring and effective connectivity of the whole brain. In both modalities, we examined the influence of cytoarchitecture on extrinsic connectivity of the DMN. Finally, we evaluated the uniqueness of the DMN relative to other large-scale functional brain networks.
Results. We discovered profound diversity of DMN cytoarchitecture. Each circumscribed subregion of the DMN contains a broad range of cytoarchitectural types, however, the spatial pattern within each subregion differs. The patterns vary in smoothness from a gradient in the parahippocampus to interdigitation in the superior frontal gyrus. We found that cytoarchitectural differentiation in the DMN aligns with its structural wiring and extrinsic information flow. The structural heterogeneity of the DMN engenders a network-level balance in communication with external and internal sources, which is distinctive, relative to other functional networks.
Conclusion. These findings suggest a novel wiring diagram of structural and functional connectivity of the DMN that is compatible with its putative role in balancing internal and external information. Furthermore, our work demonstrates the import of neuroanatomical evidence in specifying theories of functional networks.
All information about the 5th BigBrain Workshop 2021, including detailed authors information: https://go.fzj.de/BigBrainWorkshop2021

An amazing graph theoretic analysis of the C. elegans neuropeptide connectome!
Neuromodulation by peptides is essential for brain function. By comprehensively mapping neuropeptide signaling in the nematode C. elegans, Ripoll-Sánchez et al. define a dense wireless network whose organization differs in important ways from wired brain circuits. This network is a prototype for understanding neuropeptide signaling networks in larger brains.
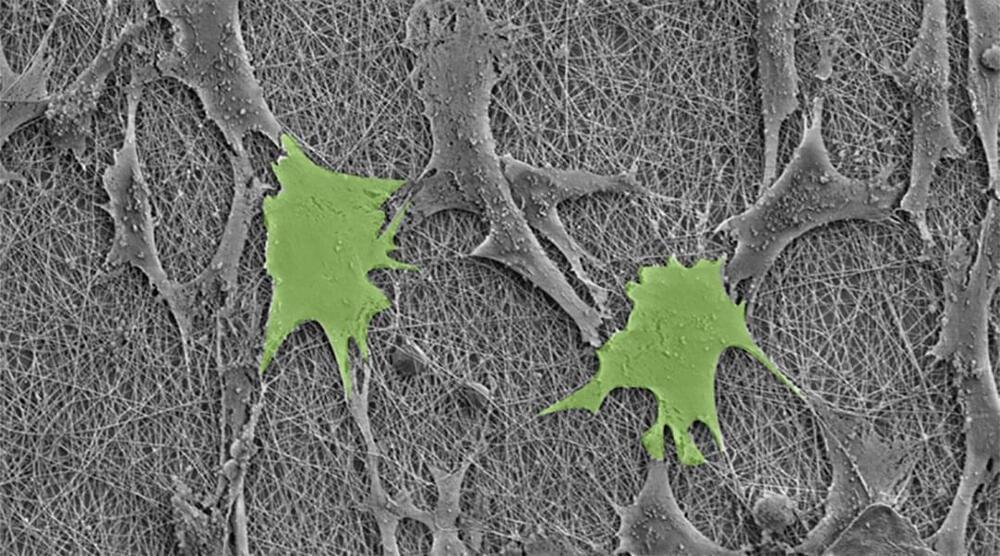
Weaving piezoelectric polymers into nanofibers reveals a surprising pathway to boost stem cell growth naturally, without external power.
Our bodies are a complex tapestry of cells, woven into tissues and organs, like bones, muscle, and skin. All these cells begin as blank slates called stem cells, which are directed to become all the unique cell types in the body by a myriad of genetic and environmental cues.
To harness the biomedical potential of stem cells, researchers have long sought ways to untangle these factors and find a recipe to efficiently grow any desired cell type. Now, expertise from textile research is helping create a new platform to achieve this goal.
Investigators from the laboratory of Ali Shilatifard, Ph.D., the Robert Francis Furchgott Professor and chair of Biochemistry and Molecular Genetics, have discovered a new repeat gene cluster sequence that is exclusively expressed in humans and non-human primates.
The discovery, detailed in a study published in Science Advances, is a breakthrough for human genome biology and has wide-ranging implications for future research in transcriptional regulation, human evolution, and the study of repetitive DNA sequences, according to the authors.
“This is an unbelievable discovery of the first elongation factor that is repeated within the human genome and is very primate-specific,” said Shilatifard, who is also director of the Simpson Querrey Institute for Epigenetics and a professor of Pediatrics.

The groundbreaking gene-editing technology known as Crispr, which acts like a molecular pair of scissors that can be used to cut and modify a DNA sequence, has moved rather quickly from the pages of scientific journals to the medical setting. Earlier this month, about three years after Jennifer Doudna and Emmanuelle Charpentier won the Nobel Prize in Chemistry for describing how bacteria’s immune system could be used as a tool to edit genes, regulators in the U.K. approved the first Crispr-based treatment for sickle cell disease and beta-thalassemia patients. The treatment, from Vertex Pharmaceuticals and Crispr Therapeutics, could be approved by the U.S. Food and Drug Administration early next month for sickle cell patients.
While many obstacles lie ahead for the nascent field, such as how to pay for treatments that typically cost more than $1 million, these regulatory approvals are just the start as newer gene-editing technologies such as base and prime editing make their way through human studies. In an interview, Prof. Doudna says the approval is “a turning point in medicine because it really shows how genome editing can be used as a one-and-done cure for disease.”
Gene editing is part of a broader therapeutic revolution that encompasses genetic and cellular medicine. The pills and injections we are all familiar with generally target proteins or pathways in the body to treat disease. With gene and cell therapy, we can now target the root cause of disease, sometimes curing patients.

Monoclonal antibodies are immune system proteins that are created in the lab. Antibodies are produced naturally by your body and help the immune system recognize germs that cause disease, such as bacteria and viruses, and mark them for destruction. Like your body’s own antibodies, monoclonal antibodies recognize specific targets.
Many monoclonal antibodies are used to treat cancer. They are a type of targeted cancer therapy, which means they are designed to interact with specific targets. Learn more about targeted therapy.
Some monoclonal antibodies are also immunotherapy because they help turn the immune system against cancer. For example, some monoclonal antibodies mark cancer cells so that the immune system will better recognize and destroy them. An example is rituximab, which binds to a protein called CD20 on B cells and some types of cancer cells, causing the immune system to kill them. B cells are a type of white blood cell.

Cleveland Clinic researchers analyzed genes and brain tissue of patients with Alzheimer’s and found that differences in brain immunometabolism – the interactions between the immune system and the ways cells create energy – may contribute to women’s increased risk for the disease and its severity.
The findings, published in Alzheimer’s and Dementia, offer important insight into developing sex-specific treatment and prevention options for Alzheimer’s disease, the sixth-leading cause of death in the United States.
“Our immune systems depend on communication between different cell types in our bodies, which are fueled by energy created from unique metabolic processes,” said Justin Lathia, Ph.D., vice chair of the Department of Cardiovascular and Metabolic Sciences and co-author on the paper. “As sex influences both the immune system and metabolic process, our study aimed to identify how all of these individual factors influence one another to contribute to Alzheimer’s disease.”
The next big advance in cancer treatment could be a vaccine that can shrink tumors and stop cancer from coming back. Among the targets for the experimental shots: melanoma, pancreatic cancer, breast cancer and lung cancer. Scientists are predicting new cancer vaccine approvals within five years (June 26) (AP Video by Manuel Valdes & Carla Johnson)
Subscribe for more Breaking News: http://smarturl.it/AssociatedPress.
Website: https://apnews.com.
Twitter: https://twitter.com/AP
Facebook: https://facebook.com/APNews.
Instagram: https://www.instagram.com/APNews/
You can license this story through AP Archive: http://www.aparchive.com/metadata/youtube/feab753e210b4468925734a2936956dd.
#cancer