Both for research and medical purposes, researchers have spent decades pushing the limits of microscopy to produce ever deeper and sharper images of brain activity, not only in the cortex but also in regions underneath such as the hippocampus. In a new study, a team of MIT scientists and engineers demonstrates a new microscope system capable of peering exceptionally deep into brain tissues to detect the molecular activity of individual cells by using sound.
“The major advance here is to enable us to image deeper at single-cell resolution,” said neuroscientist Mriganka Sur, a corresponding author along with mechanical engineering Professor Peter So and principal research scientist Brian Anthony. Sur is the Paul and Lilah Newton Professor in The Picower Institute for Learning and Memory and the Department of Brain and Cognitive Sciences at MIT.
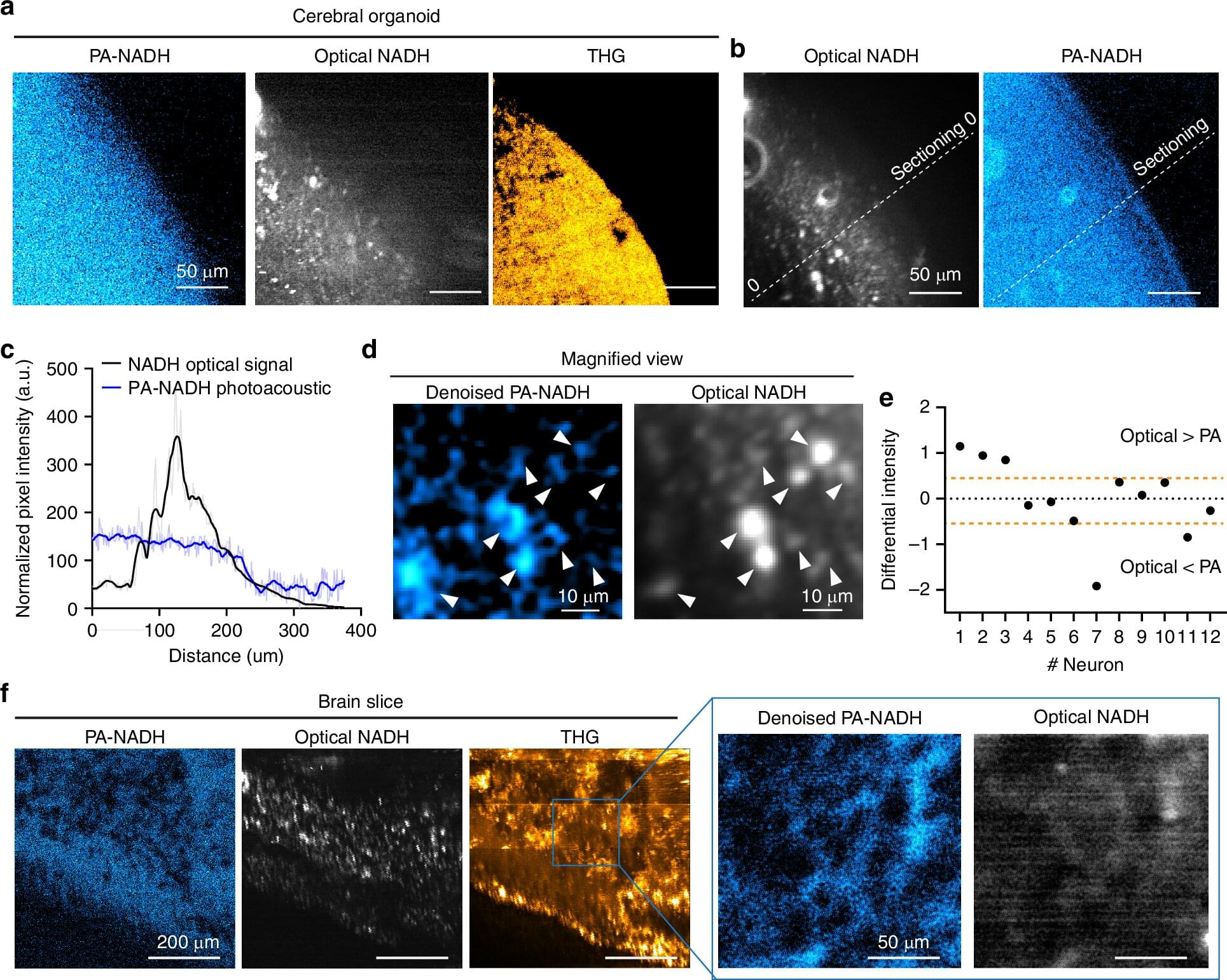
In the journal Light: Science and Applications, the team demonstrates that they could detect NAD℗H, a molecule tightly associated with cell metabolism in general, and electrical activity in neurons in particular, all the way through samples such as a 1.1 mm “cerebral organoid,” a 3D-mini brain-like tissue generated from human stem cells, and a 0.7 mm thick slice of mouse brain tissue.