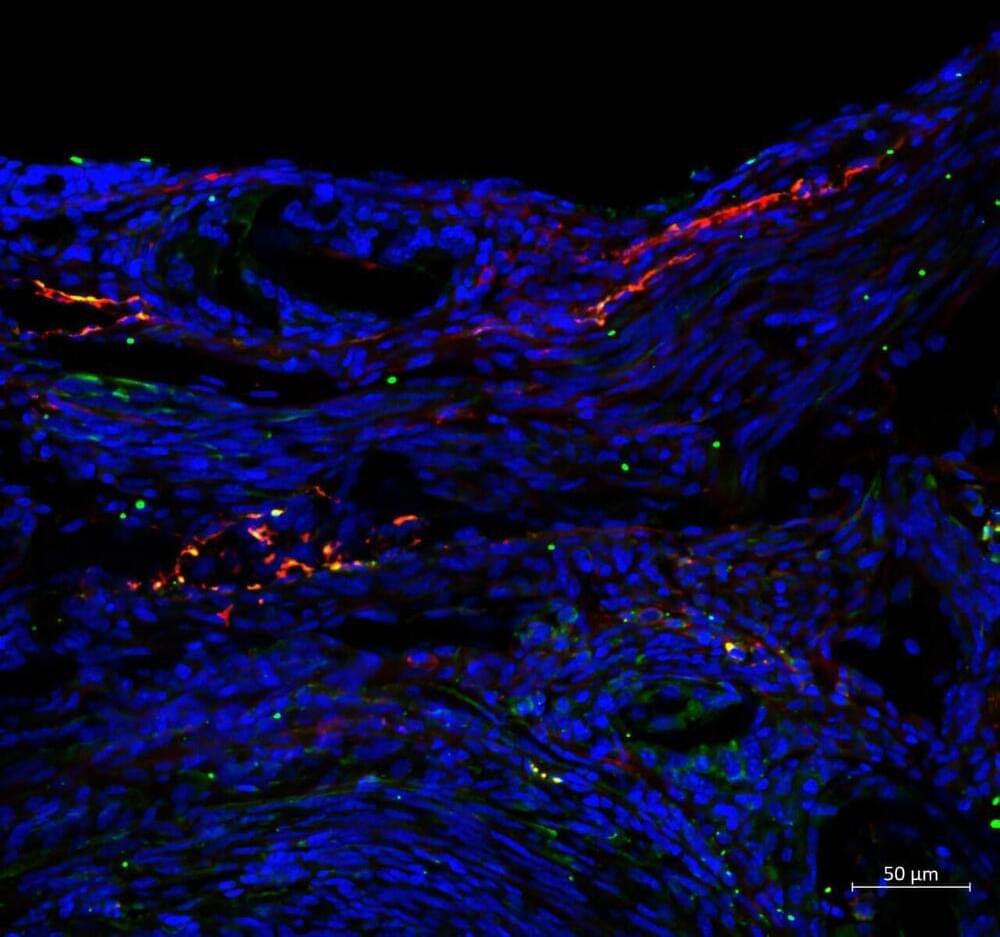
We use our lips to talk, eat, drink, and breathe; they signal our emotions, health, and aesthetic beauty. It takes a complex structure to perform so many roles, so lip problems can be hard to repair effectively. Basic research is essential to improving these treatments, but until now, models using lip cells—which perform differently to other skin cells—have not been available.
In a study published in Frontiers in Cell and Developmental Biology, scientists report the successful immortalization of donated lip cells, allowing for the development of clinically relevant lip models in the lab. This proof-of-concept, once expanded, could benefit thousands of patients.
“The lip is a very prominent feature of our face,” said Dr. Martin Degen of the University of Bern.