What impact can cannabis have on opioid use? This is what a recent study published in JAMA Internal Medicine hopes to address as an international team of r | Cannabis Sciences


Get Our Newsletter (It’s Free): https://www.optispan.life/
Dive into the future of longevity research with Dr. Ben Blue, CEO of Ora Biomedical. We explore how Ora’s high-throughput “Wormbot” platform is conducting the world’s largest unbiased search for longevity interventions, moving beyond the narrow focus on established pathways like mTOR.
In this episode, we discuss:
• The ambitious Million Molecule Challenge and why it could revolutionize the field.
• Surprising discoveries already made, including molecules that outperform rapamycin.
• Ora’s strategic pivot to radiation resistance, with applications for astronauts, pilots, and human health.
• How their data-driven approach is uncovering interventions for resilience against toxins and other stresses.
• The journey from worm models to potential clinical trials and what’s next for the company.
Learn how Ora is scaling drug discovery to tackle aging and age-related diseases.
https://twitter.com/OraBiomedical.
https://www.linkedin.com/company/ora-biomedical-inc/
This video was produced by One Billion Media, an agency that specializes in YouTube virality for health brands and experts. Learn more about their work here:


Heat is one therapy to help damage and kill cancer cells, according to the National Cancer Institute. Steam offers a targeted way to deliver heat into the body, according to Abreu.
Before the procedure, physicians use magnetic resonance imaging (MRI) to locate the tumor cells in the patient’s prostate. During the procedure, doctors use an ultrasound and prostate mapping to guide a thin catheter through the patient’s urethra and into the area of the prostate where the tumor is located.
Once the catheter is positioned, a fine needle is deployed in the tumor. Doctors then release a quick, targeted 10-second burst of steam from the needle, and more bursts as needed, to destroy the tumor.
‘This procedure is thought to be much gentler on the body than traditional therapies and is designed to target the cancerous tissue within the prostate,’ said Abreu. ‘We are exploring if steam may be effective at destroying cancer cells without damaging the surrounding organs.’
B-roll video available for download below.

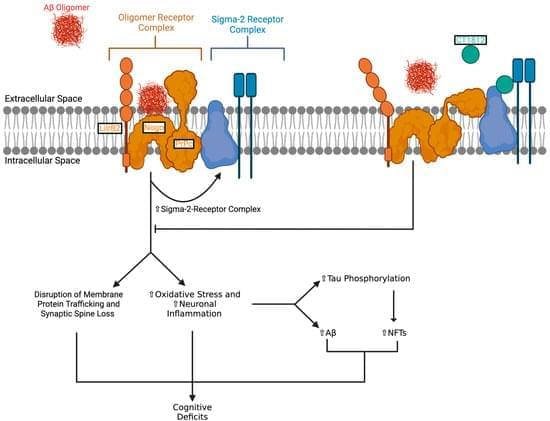
Alzheimer’s disease (AD) is a neurodegenerative disease marked by the accumulation of toxic amyloid-beta (Aβ) oligomers. These oligomers are thought to cause synaptic dysfunction and contribute to neurodegeneration. CT1812 is a small-molecule sigma-2 receptor antagonist that is currently being investigated and tested as a potential disease-modifying treatment for AD. CT1812 acts by displacing Aβ oligomers into the cerebrospinal fluid and preventing their interaction with receptors on neurons. Preclinical studies and early clinical trials of CT1812 show promising results and provide evidence for its potential to slow AD progression. This review outlines the role of Aβ oligomers in AD, CT1812’s mechanism of action, and the effectiveness and limitations of CT1812 based on preclinical and clinical studies.
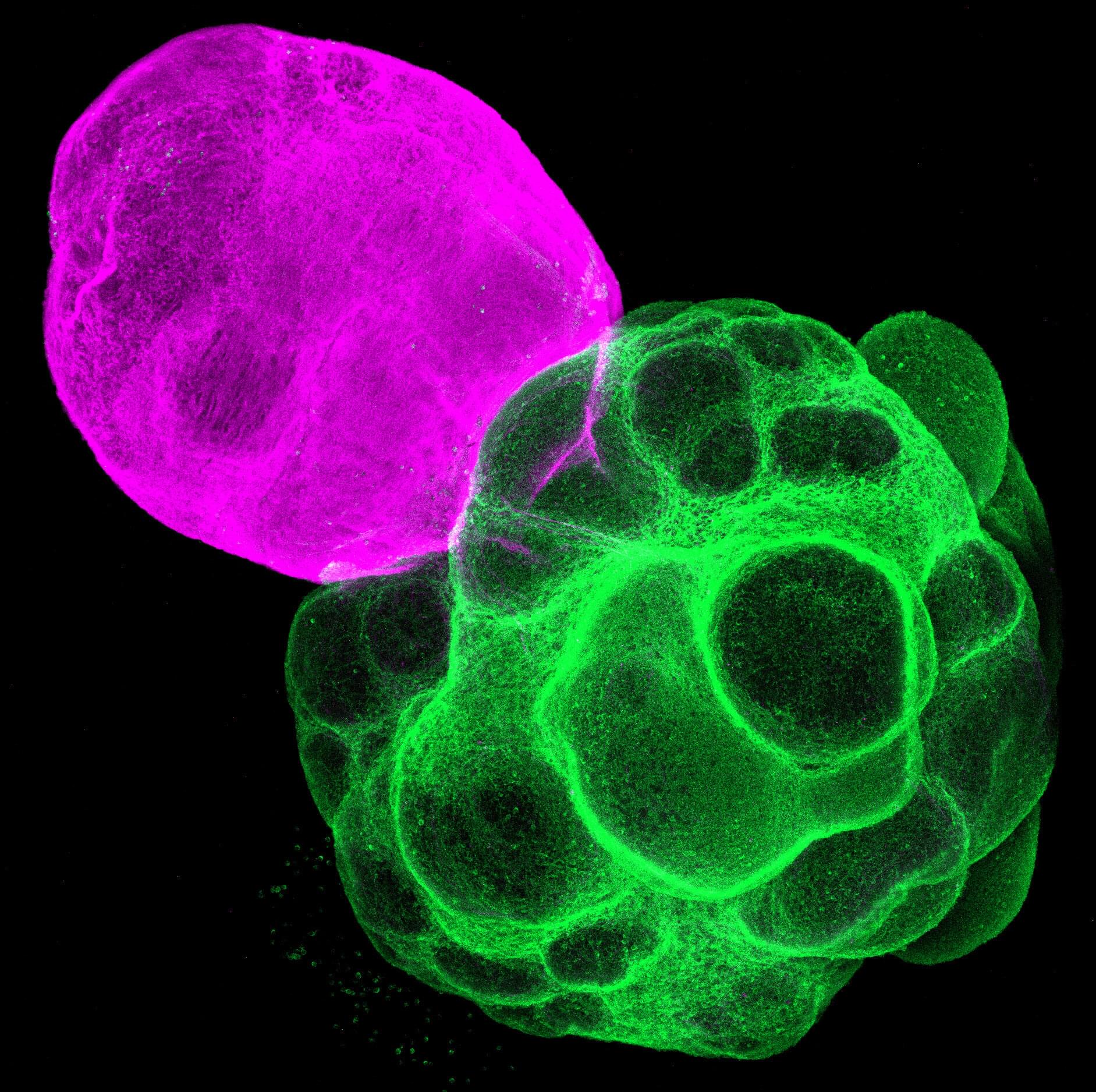
A Japanese research team has successfully reproduced the human neural circuit in vitro using multi-region miniature organs known as assembloids, which are derived from induced pluripotent stem (iPS) cells. With this circuit, the team demonstrated that the thalamus plays a crucial role in shaping cell type-specific neural circuits in the human cerebral cortex.
These findings were published in the journal Proceedings of the National Academy of Sciences.
Our brain’s cerebral cortex contains various types of neurons, and effective communication among these neurons and other brain regions is crucial for activating functions like perception and cognition.
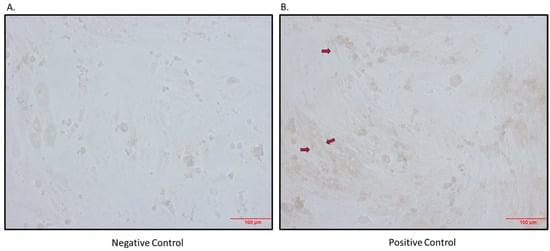
The hyperactivation of the sympathetic nervous system (SNS) is linked to obesity, hypertension, and type 2 diabetes, which are characterized by elevated norepinephrine (NE) levels. Previous research has shown increased sodium-dependent glucose cotransporter 1 (SGLT1) protein levels in kidneys of hypertensive rodents, prompting investigation into the expression of SGLT1 in various tissues, such as skeletal muscle. This study aimed to assess (i) whether skeletal muscle cells and tissue express SGLT1 and SGLT2 proteins; (ii) if NE increases SGLT1 levels in skeletal muscle cells, and (iii) whether the skeletal muscle of neurogenically hypertensive mice exhibits increased SGLT1 expression. We found that (i) skeletal muscle cells and tissue are a novel source of the SGLT2 protein and that (ii) NE significantly elevated SGLT1 levels in skeletal muscle cells. As SGLT2 inhibition (SGLT2i) with Empagliflozin increased SGLT1 levels, in vivo studies with the dual inhibitor SGLT1/2i, Sotagliflozin were warranted. The treatment of neurogenically hypertensive mice using Sotagliflozin significantly reduced blood pressure. Our findings suggest that SNS activity upregulates the therapeutic target, SGLT1, in skeletal muscle, potentially worsening cardiometabolic control. As clinical trial data suggest cardiorenal benefits from SGLT2i, future studies should aim to utilize SGLT1i by itself, which may offer a therapeutic strategy for conditions with heightened SNS activity, such as hypertension, diabetes, and obesity.
This case-control study found that adults with schizophrenia had significantly greater frontal cortex serotonin release than healthy controls, and greater release correlated with more severe negative symptoms.
Question Is serotonin release altered in vivo in schizophrenia, and is it associated with negative symptom severity?
Findings In this case-control neuroimaging study that included 54 adults, frontal cortex serotonin release was significantly greater in the 26 people with DSM-5 schizophrenia compared with 28 matched healthy controls. In schizophrenia, greater frontal cortex serotonin release was associated with more severe baseline negative symptoms.
Meaning Findings suggest that serotonergic dysfunction in the pathophysiology of schizophrenia was associated with negative symptoms, identifying the regulation of serotonergic neurotransmission as a potential target to treat negative symptoms.