Metabolic control of antitumor Immunity in neuroblastoma👇
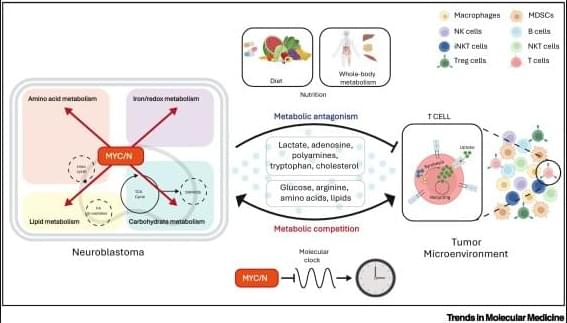
✅Distinct metabolic TMEs in MNA vs non-MNA tumors Neuroblastoma (NB) tumors display markedly different tumor microenvironments depending on MYCN amplification (MNA) status. MYCN acts as a key driver of metabolic remodeling, reshaping how nutrients and metabolites are distributed within the TME.
✅MYCN-amplified NB: immunosuppressive metabolism In MNA neuroblastoma, tumor cells aggressively consume shared metabolites such as glucose, glutamine, methionine, cysteine, and lipids. This metabolic competition deprives infiltrating T cells of essential nutrients, promoting T cell exhaustion and dysfunction. In parallel, MNA tumor cells release antagonistic metabolites, including lactate, adenosine, and cholesterol, which further suppress TCR signaling, proliferation, and effector functions.
✅T cell exhaustion and impaired tumor killing Within the MNA TME, T cells exhibit exhausted phenotypes characterized by impaired proliferation, altered STAT5 signaling, and reduced cytotoxic activity. This metabolic and signaling imbalance leads to ineffective immune-mediated tumor killing.
✅Non-MNA NB: permissive immune landscape In contrast, non-MNA neuroblastomas do not impose the same metabolic constraints. Nutrient availability is better preserved, allowing cytotoxic T cells to infiltrate tumors, maintain effector functions, and induce effective tumor cell death.
✅Biological and therapeutic implications These findings highlight metabolism as a central regulator of antitumor immunity in neuroblastoma. Targeting MYCN-driven metabolic pathways may help restore T cell function and improve the efficacy of immunotherapies, particularly in MYCN-amplified disease.