Organic electrochemical transistors (OECTs) are neuromorphic transistors made of carbon-based materials that combine both electronic and ionic charge carriers. These transistors could be particularly effective solutions for amplifying and switching electronic signals in devices designed to be placed on the human skin, such as smart watches, trackers that monitor physiological signals and other wearable technologies.
In contrast with conventional neuromorphic transistors, OECTs could operate reliably in wet or humid environments, which would be highly advantageous for both medical and wearable devices. Despite their potential, most existing OECTs are based on stiff materials, which can reduce the comfort of wearables and thus hinder their large-scale deployment.
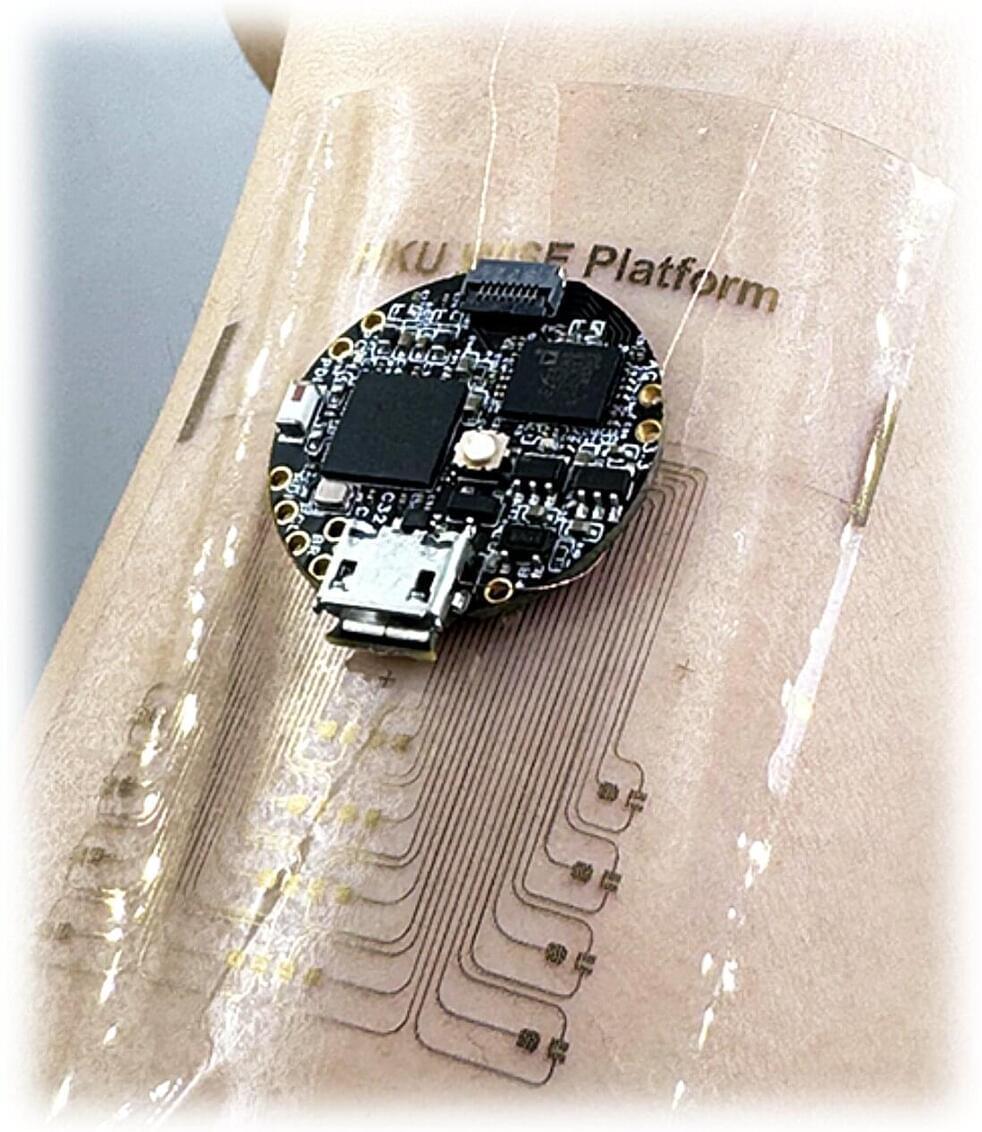
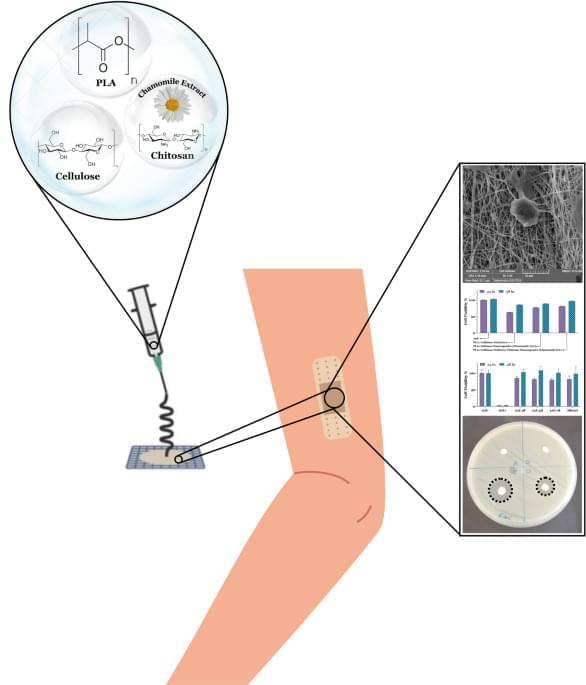
Researchers at the University of Hong Kong have developed a new wearable device based on stretchable OECTs that can both perform computations and collect signals from the surrounding environment. Their proposed system, presented in a paper published in Nature Electronics, could be used to realize in-sensor edge computing on a flexible wearable device that is comfortable for users.