A new study investigated the brain circuits involved in psychosis—a condition characterized by delusions, hallucinations, disorganized thinking and detachment from reality.
Andrew Pines, MD, MA, a resident in the Department of Psychiatry at Brigham and Women’s Hospital and a researcher in the Center for Brain Circuit Therapeutics, is the lead author of the paper published in JAMA Psychiatry titled “Mapping Lesions That Cause Psychosis to a Human Brain Circuit and Proposed Stimulation Target.”
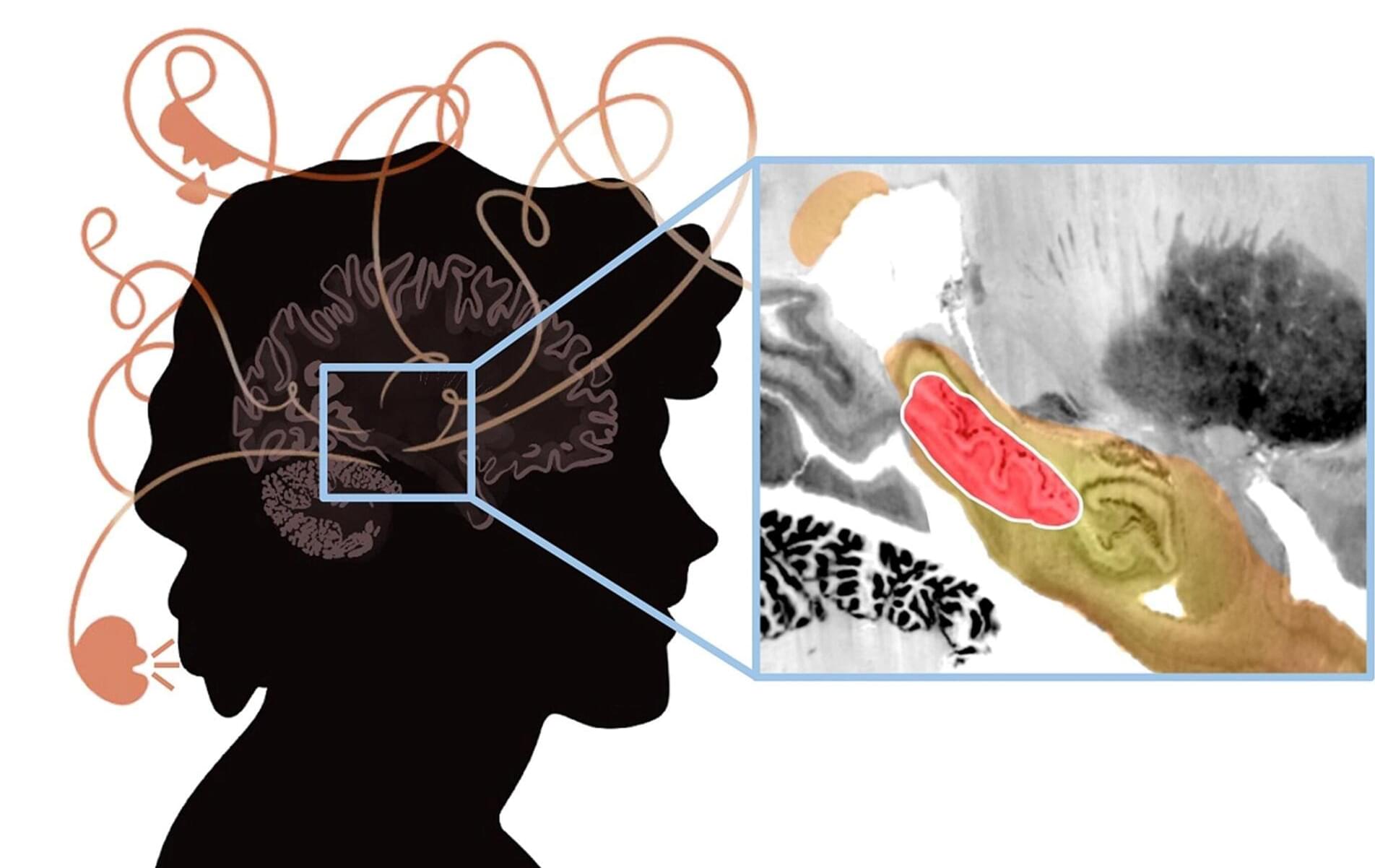
Psychosis is the classic symptom of schizophrenia, a serious mental illness that causes marked disability in otherwise young and healthy patients. The researchers analyzed published cases in which focal brain damage caused psychosis, with the idea that if damaging a brain circuit causes a symptom, then mapping that circuit might tell us about how to treat that symptom.