An investigational drug for glioblastoma more than doubled survival and progression-free time compared to standard rates. | Drug Discovery And Development

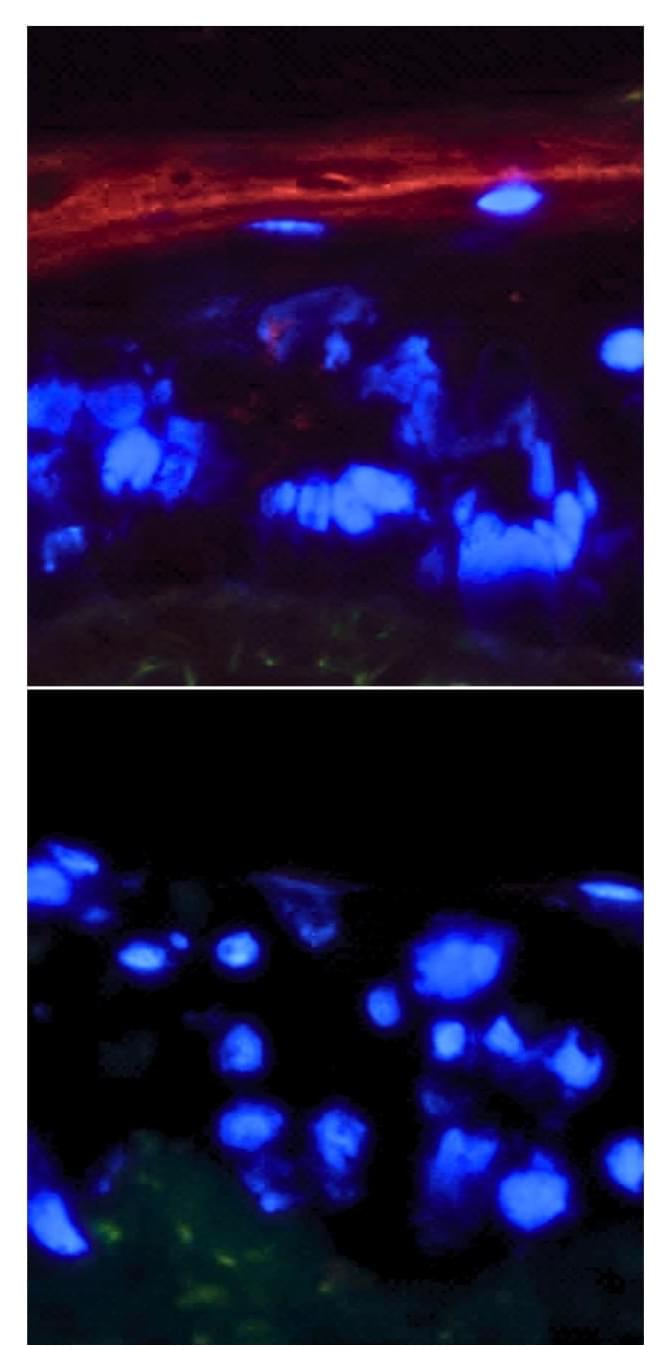
As our bodies grow, cells proliferate to form tissues, and cells frequently have to be repaired or replaced throughout life. But the genome can also become less stable over time, or may pick up mutations that can lead to disease; these and other processes can cause cells to enter a state in which they stop dividing, known as senescence. Senescent cells become more common as we age. There also tends to be more inflammation as we age, but the link between increasing instability in the genome and inflammation is not well understood. Now scientists have reported a direct connection between DNA instability and inflammation in senescent cells. The findings have been reported in Nature Communications.
“In addition to no longer growing and proliferating, the other hallmark of senescent cells is that they have this inflammatory program causing them to secrete inflammatory molecules,” noted senior study author Peter Adams, Ph.D., director and professor of the Cancer Genome and Epigenetics Program at Sanford Burnham Prebys.

University of Queensland researchers are designing nanotechnology they believe could improve how we treat the most aggressive form of breast cancer.
Professor Chengzhong (Michael) Yu and his team are developing novel nanoparticles that could dramatically increase the effectiveness of immunotherapies when treating triple-negative breast cancer (TNBC).
TNBC is aggressive, fast-growing and accounts for 30 per cent of all breast cancer deaths in Australia each year, despite making up only 10 to 15 per cent of new cases.
Professor Yu, from UQ’s Australian Institute for Bioengineering and Nanotechnology (AIBN), said a new solution was needed because TNBC cancer cells lacked the proteins targeted by some of the treatments used against other cancers.
UQ researchers are designing nanotechnology they believe could improve how we treat the most aggressive form of breast cancer.

A cybersecurity incident affecting nearly half a million people has exposed personal, financial and medical information.
The mobility and assistive solutions provider Numotion says 494,000 customers are affected by a data breach witnessed between September 2nd, 2024, and November 18th, 2024, reports Security Week.
Numotion says an unknown entity managed to access the email accounts of the firm’s employees without authorization several times.
Glucose is life’s main energy source. But a Stanford Medicine study reveals a surprising role as a master manipulator of tissue maturation, hinting at its importance in diabetes and cancer.
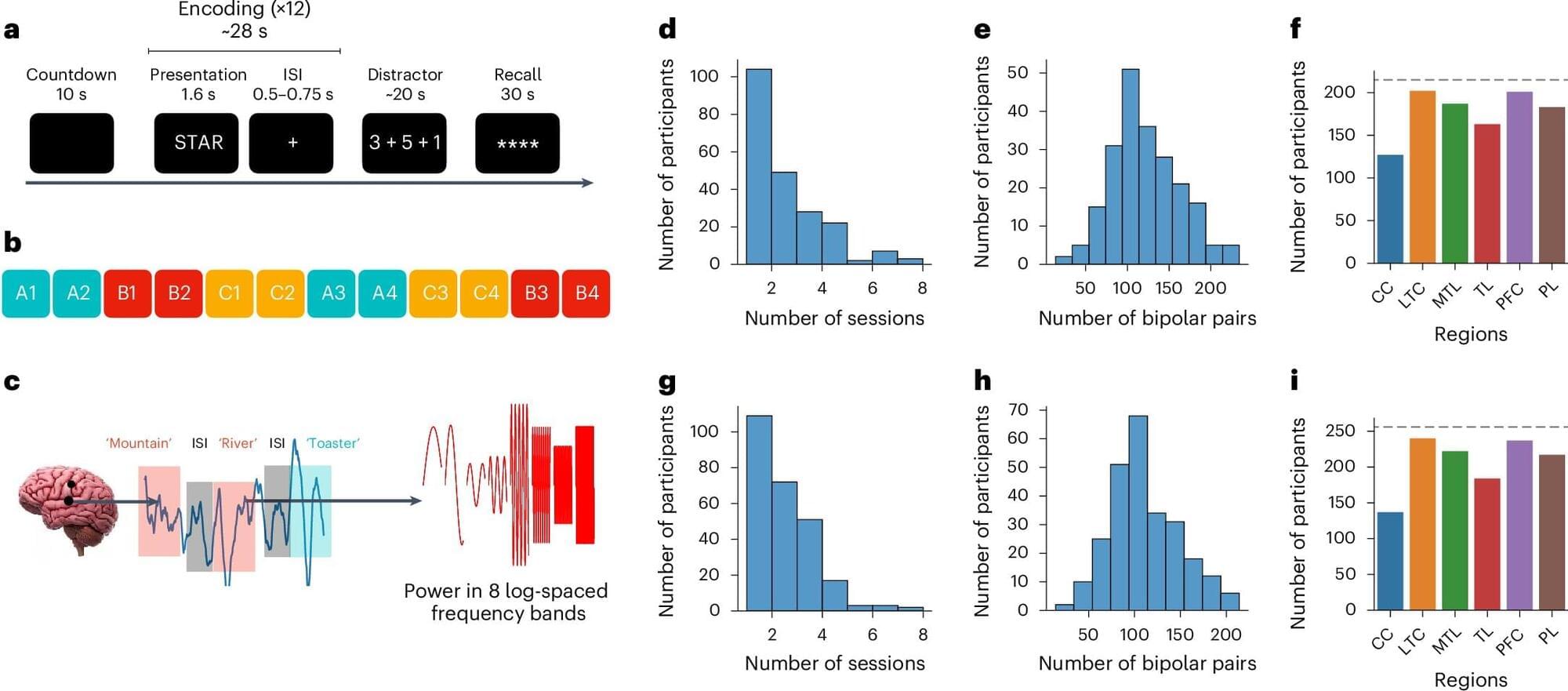
Past neuroscience and psychology studies have shown that after the human brain encodes specific events or information, it can periodically reactivate them to facilitate their retention, via a process known as memory consolidation. The reactivation of memories has been specifically studied in the context of sleep or rest, with findings suggesting that during periods of inactivity, the brain reactivates specific memories, allowing people to remember them in the long term.
Researchers at the University of Pennsylvania and other institutions in the United States recently conducted a study exploring the possibility that the brain engages in a similar reactivation process during wakefulness to store important information for shorter periods of time. Their findings, published in Nature Neuroscience, suggest that the spontaneous reactivation of specific stimuli in the brain during the brief intervals between their encoding predicts the accuracy with which people remember them at the end of a memory task.
“Mike Kahana and I were both quite interested in the long history of thinking about rehearsal and its effects on the way in which people later recalled things,” Dr. David Halpern, the first author of the paper, told Medical Xpress. “Rehearsal is challenging to study since people often do it without any overt behavior (unless we ask them to rehearse out loud).”
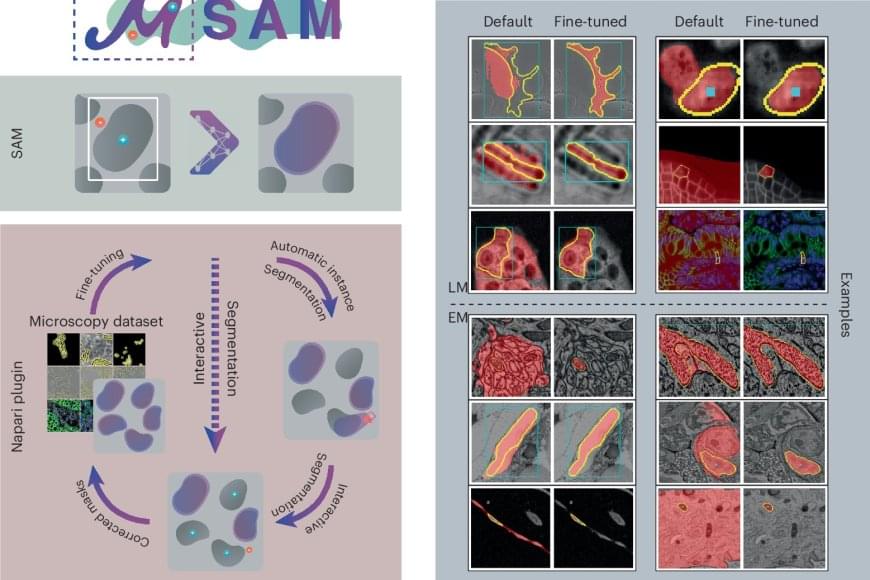
To adapt the existing software to microscopy, the research team first evaluated it on a large set of open-source data, which showed the model’s potential for microscopy segmentation. To improve quality, the team retrained it on a large microscopy dataset. This dramatically improved the model’s performance for the segmentation of cells, nuclei and tiny structures in cells known as organelles.
The team then created their software, μSAM, which enables researchers and medical doctors to analyze images without the need to first manually paint structures or train a specific AI model. The software is already in wide use internationally, for example to analyze nerve cells in the ear as part of a project on hearing restoration, to segment artificial tumor cells for cancer research, or to analyze electron microscopy images of volcanic rocks.
“Analyzing cells or other structures is one of the most challenging tasks for researchers working in microscopy and is an important task for both basic research in biology and medical diagnostics,” says the author.
Identifying and delineating cell structures in microscopy images is crucial for understanding the complex processes of life. This task is called “segmentation” and it enables a range of applications, such as analysing the reaction of cells to drug treatments, or comparing cell structures in different genotypes. It was already possible to carry out automatic segmentation of those biological structures but the dedicated methods only worked in specific conditions and adapting them to new conditions was costly.
An international research team has now developed a method by retraining the existing AI-based software Segment Anything on over 17,000 microscopy images with over 2 million structures annotated by hand.
Their new model is called Segment Anything for Microscopy and it can precisely segment images of tissues, cells and similar structures in a wide range of settings. To make it available to researchers and medical doctors, they have also created μSAM, a user-friendly software to “segment anything” in microscopy images. Their work was published in Nature Methods.