Researchers have developed a personalized blood test that may offer a faster, less invasive way to track high-grade glioma progression.

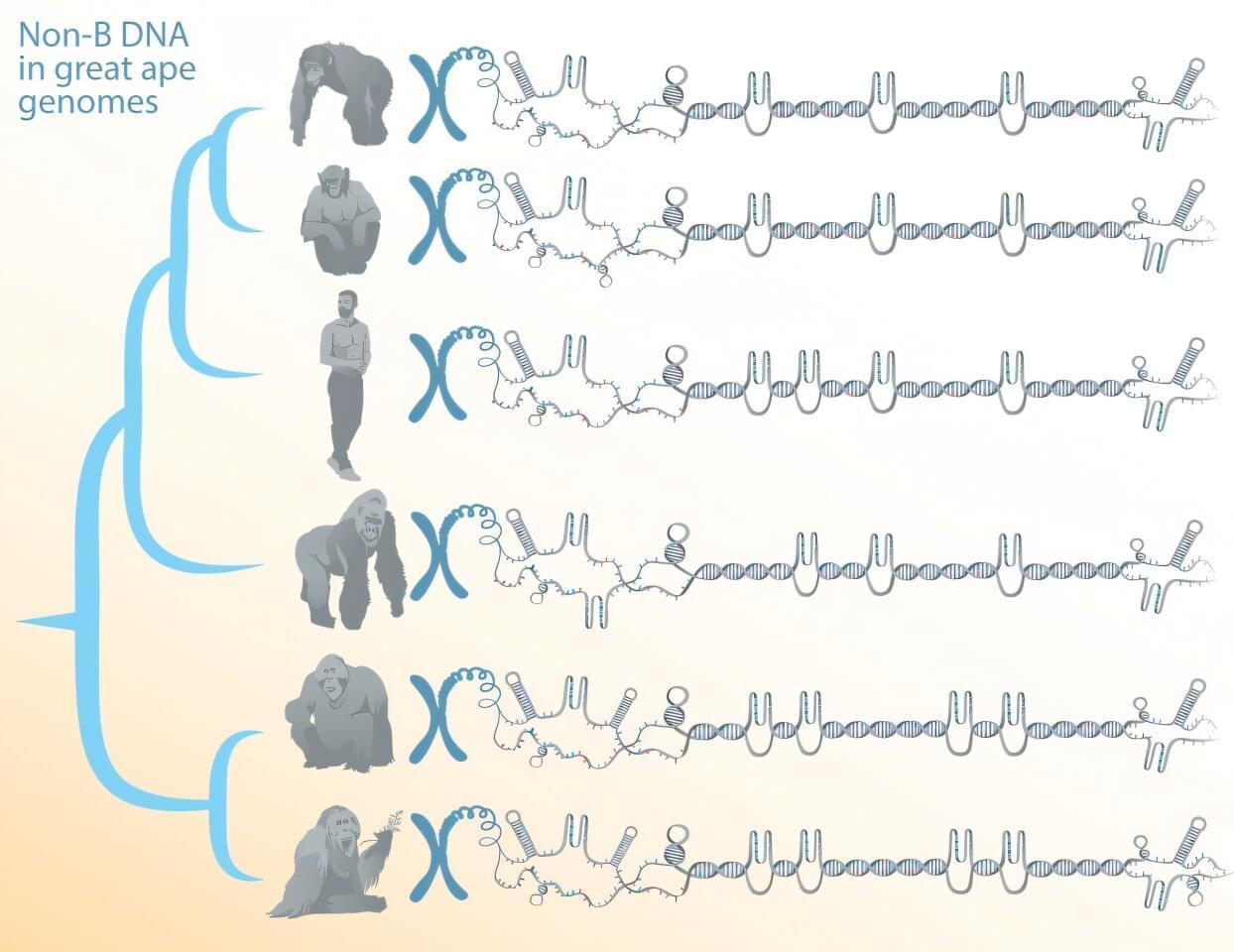
Certain DNA sequences can form structures other than the canonical double helix. These alternative DNA conformations—referred to as non-B DNA—have been implicated as regulators of cellular processes and of genome evolution, but their DNA tends to be repetitive, which until recently made reliably reading and assembling their sequences difficult.
Now, a team of researchers, led by Penn State biologists, has comprehensively predicted the location of non-B DNA structures in great apes. It’s the first step in understanding the functions and evolution of such structures, known to contribute to genetic diseases and cancer, the team said.
The work depends on newly available telomere-to-telomere (T2T), or end-to-end, genomes of humans and other great apes that overcame sequencing and assembly difficulties associated with repetitive DNA to fill in any remaining gaps in the genomes. A paper describing the study, which shows that non-B DNA is enriched in the newly sequenced segments of the genomes and suggests potential new functions, was published in the journal Nucleic Acids Research.

Patients in the early stages of psychosis respond to treatments differently than those who have developed a chronic version of the disorder. Understanding the neurobiological changes from early to chronic stages is essential for developing targeted prevention and treatment strategies. But how symptoms change during this transition—and what role the brain plays—is unclear.
Researchers at Yale School of Medicine (YSM) have now examined patients with early and chronic forms of psychosis to map symptom evolution and identify relevant brain networks. They published their findings in the journal Neuropsychopharmacology.
“We are interested in how psychosis and psychiatric disorders develop,” says Maya Foster, first author of the study and a Ph.D. student in the lab of Dustin Scheinost, Ph.D., associate professor of radiology and biomedical imaging at YSM.
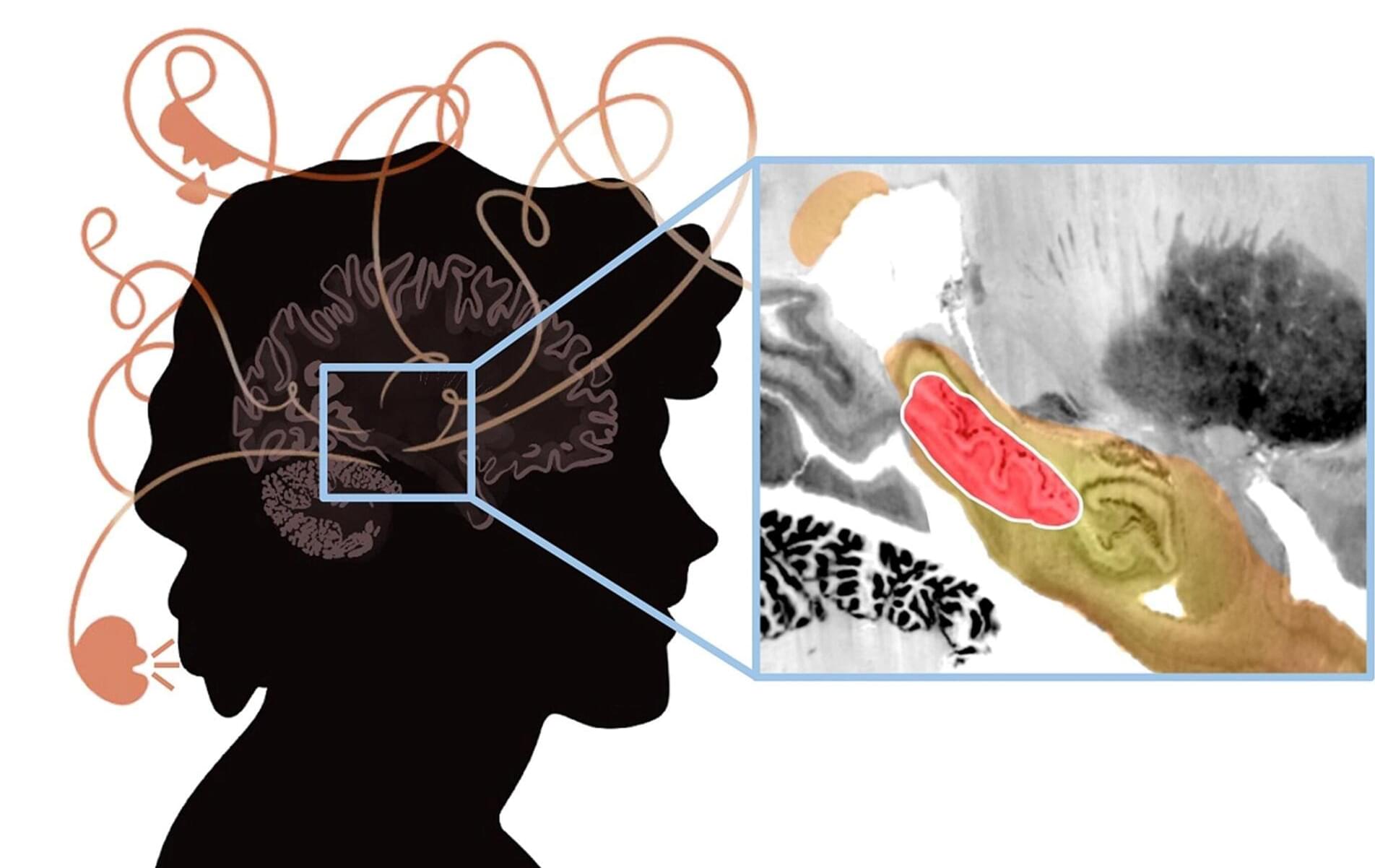
A new study investigated the brain circuits involved in psychosis—a condition characterized by delusions, hallucinations, disorganized thinking and detachment from reality.
Andrew Pines, MD, MA, a resident in the Department of Psychiatry at Brigham and Women’s Hospital and a researcher in the Center for Brain Circuit Therapeutics, is the lead author of the paper published in JAMA Psychiatry titled “Mapping Lesions That Cause Psychosis to a Human Brain Circuit and Proposed Stimulation Target.”
Psychosis is the classic symptom of schizophrenia, a serious mental illness that causes marked disability in otherwise young and healthy patients. The researchers analyzed published cases in which focal brain damage caused psychosis, with the idea that if damaging a brain circuit causes a symptom, then mapping that circuit might tell us about how to treat that symptom.

For individuals with Alzheimer disease (AD), the emergence of psychosis is associated with elevations in levels of plasma tau phosphorylated at threonine 181 (p-tau181), according to a study published online June 26 in JAMA Psychiatry.
Jesus J. Gomar, Ph.D., and Jeremy Koppel, M.D., from the Feinstein Institutes for Medical Research in Manhasset, New York, examined the longitudinal dynamics of p-tau181 and neurofilament light chain protein (NfL) levels in association with the emergence of psychotic symptoms. Patients with mild cognitive impairment (MCI) and AD (with psychosis [AD+P] and without psychosis [AD−P]) and participants who were cognitively unimpaired (CU) were compared at baseline.
For the longitudinal analysis, participants with MCI and AD were categorized into those with evidence of psychosis at baseline and those who showed incidence of psychosis over the course of the study. The cohort included 752 participants with AD and 424 CU participants.
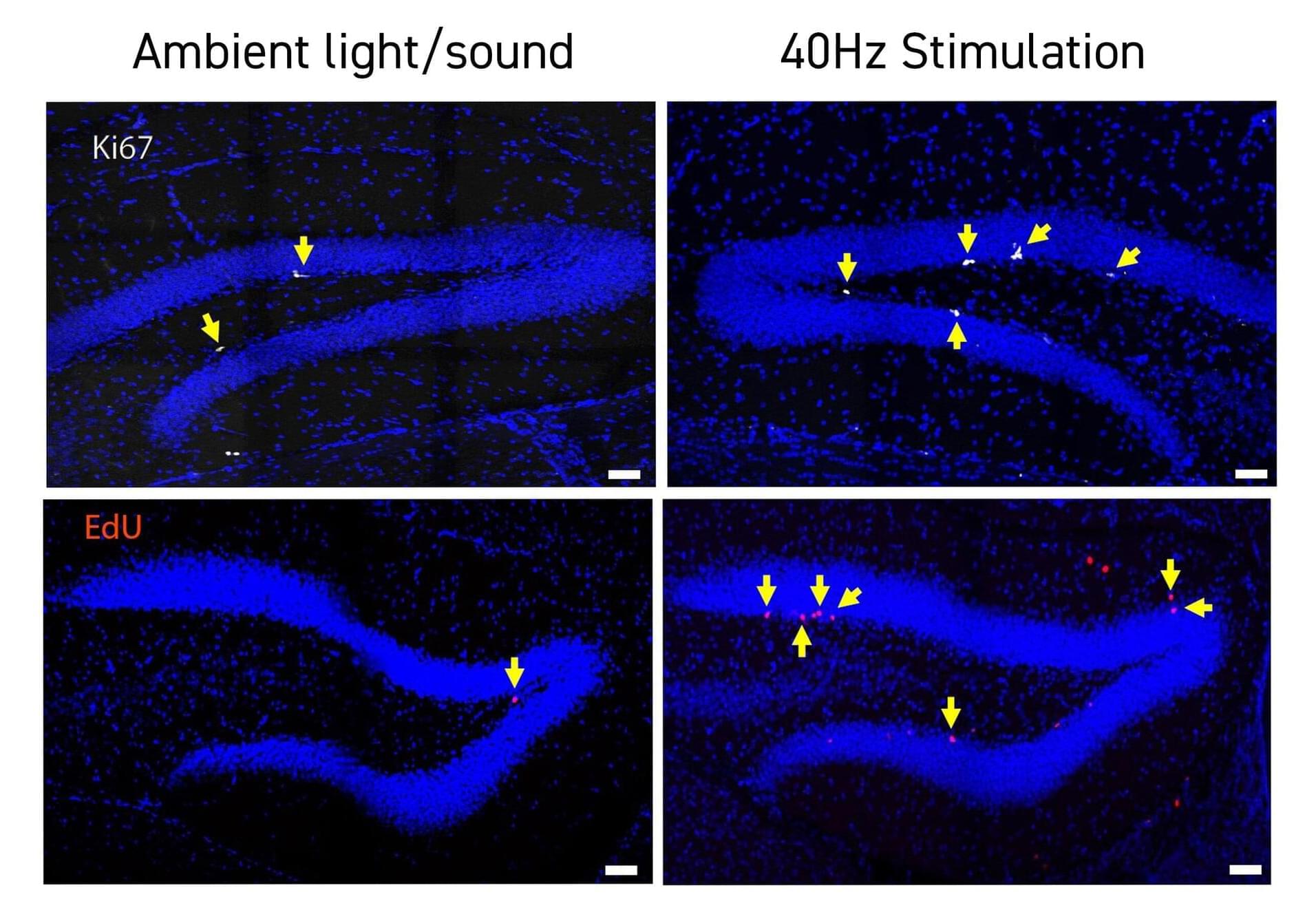
Studies by a growing number of labs have identified neurological health benefits from exposing human volunteers or animal models to light, sound and/or tactile stimulation at the brain’s “gamma” frequency rhythm of 40Hz. In the latest such research at The Picower Institute for Learning and Memory and Alana Down Syndrome Center at MIT, scientists found that 40Hz sensory stimulation improved cognition and circuit connectivity and encouraged the growth of new neurons in mice genetically engineered to model Down syndrome.
Li-Huei Tsai, Picower Professor at MIT and senior author of the new study in PLOS ONE, said that the results are encouraging but also cautioned that much more work is needed to test whether the method, called GENUS (for Gamma Entrainment Using Sensory Stimulation), could provide clinical benefits for people with Down syndrome. Her lab has begun a small study with human volunteers at MIT.
“While this work, for the first time, shows the beneficial effects of GENUS on Down syndrome using an imperfect mouse model, we need to be cautious as there is not yet data showing whether this also works in humans,” said Tsai, who directs The Picower Institute and The Alana Center, and is a member of MIT’s Brain and Cognitive Sciences faculty.
Learning and memory impairments in a Down syndrome mouse model were reversed by correcting expression of a gene that influences the generation of new neurons in the brain. The finding could pave the way to treat the cognitive impairment associated with the syndrome in humans.
Adult neurogenesis is the process of generating new neurons in the adult brain. Defects in this process have been observed in various animal models of neurological disorders including schizophrenia, depression, Parkinson’s disease, Alzheimer’s disease, and neurodevelopmental disorders such as Down syndrome. But the precise cellular and molecular mechanisms underlying adult neurogenesis and their links to neurological disorders are not well understood.
Molecular neurobiologist Kyung-Tai Min at Korea’s Ulsan National Institute of Science and Technology and his colleagues found that interactions between a gene called the Down syndrome critical region 1 (DSCR1) and two other molecules, TET1 and miRNA-124, were necessary for adult neurogenesis and were important in learning and memory.
An experiment that could confirm the fifth state of matter in the universe—and change physics as we know it—has been published in a new paper from the University of Portsmouth.
Physicist Dr. Melvin Vopson has already published research suggesting that information has mass and that all elementary particles, the smallest known building blocks of the universe, store information about themselves, similar to the way humans have DNA.
Now, he has designed an experiment—which if proved correct—means he will have discovered that information is the fifth form of matter, alongside solid, liquid, gas and plasma.

Despite testing negative for rheumatoid arthritis, doctors diagnosed her with the condition after four months of visits.
However, the 40-year-old, who owns a marketing company, soon experienced excruciating stomach pains and a dramatic 14-pound weight loss within a month, with doctors attributing it to acid reflux.
Unsatisfied and desperate for answers, Bannon turned to the AI chatbot developed by OpenAI for a potential diagnosis, which she had been using for work.