npj Metabolic Health and Disease –Zenodo https://zenodo.org/uploads/14687361 (2025).
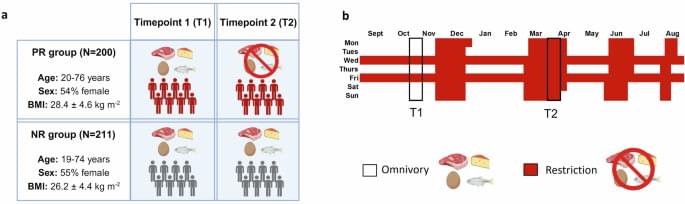

npj Metabolic Health and Disease –Zenodo https://zenodo.org/uploads/14687361 (2025).
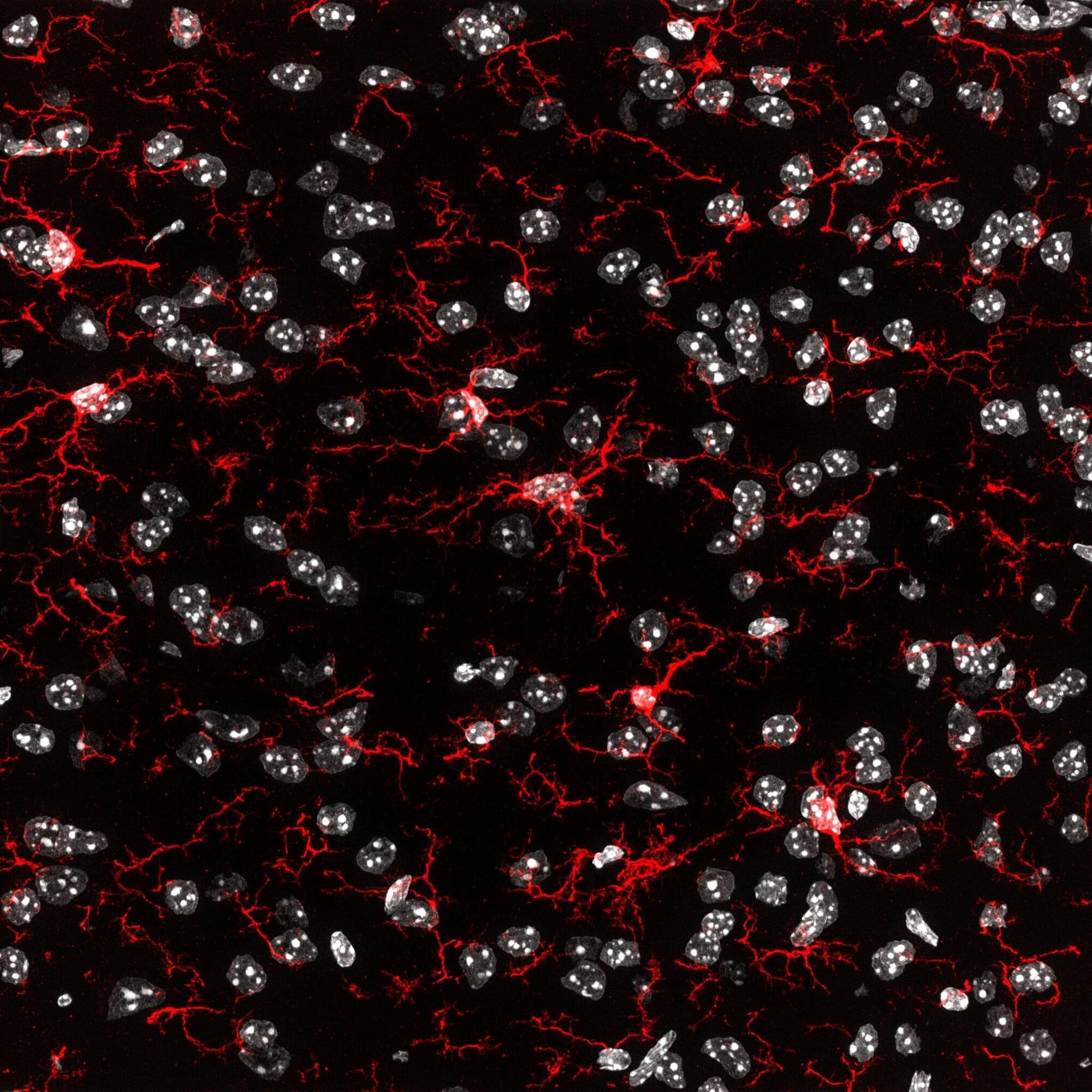
An international research team led by Professor Kiavash Movahedi from the Brussels Center for Immunology at the Vrije Universiteit Brussel has published unexpected results in the journal Immunity. Their study sheds new light on the possibility of effectively replacing defective microglia—the brain’s immune cells—marking a potential breakthrough in the treatment of neurodegenerative diseases such as Alzheimer’s and Parkinson’s.
Microglia are essential for healthy brain function. Defective microglia are increasingly linked to the development of neurodegenerative disorders.
“Microglia are unique,” says Prof. Movahedi. “They originate early in embryonic development and maintain themselves throughout life without being replaced by new cells from the blood. That makes them special, but also vulnerable.”
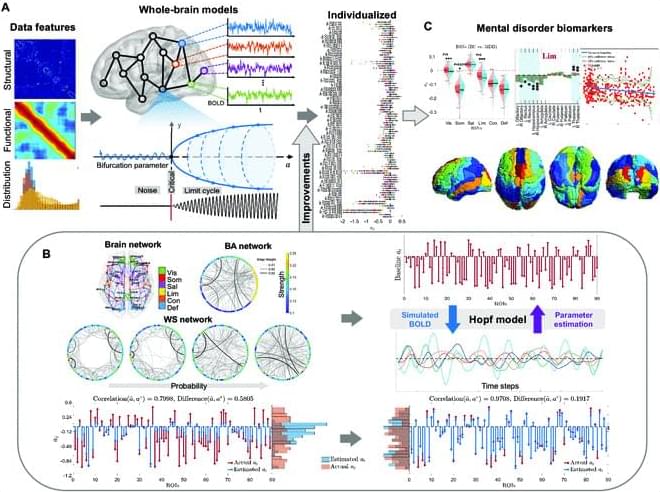
The Hopf whole-brain model, based on structural connectivity, overcomes limitations of traditional structural or functional connectivity-focused methods by incorporating heterogeneity parameters, quantifying dynamic brain characteristics in healthy and diseased states. Traditional parameter fitting techniques lack precision, restricting broader use. To address this, we validated parameter fitting methods using simulated networks and synthetic models, introducing improvements such as individual-specific initialization and optimized gradient descent, which reduced individual data loss. We also developed an approximate loss function and gradient adjustment mechanism, enhancing parameter fitting accuracy and stability.
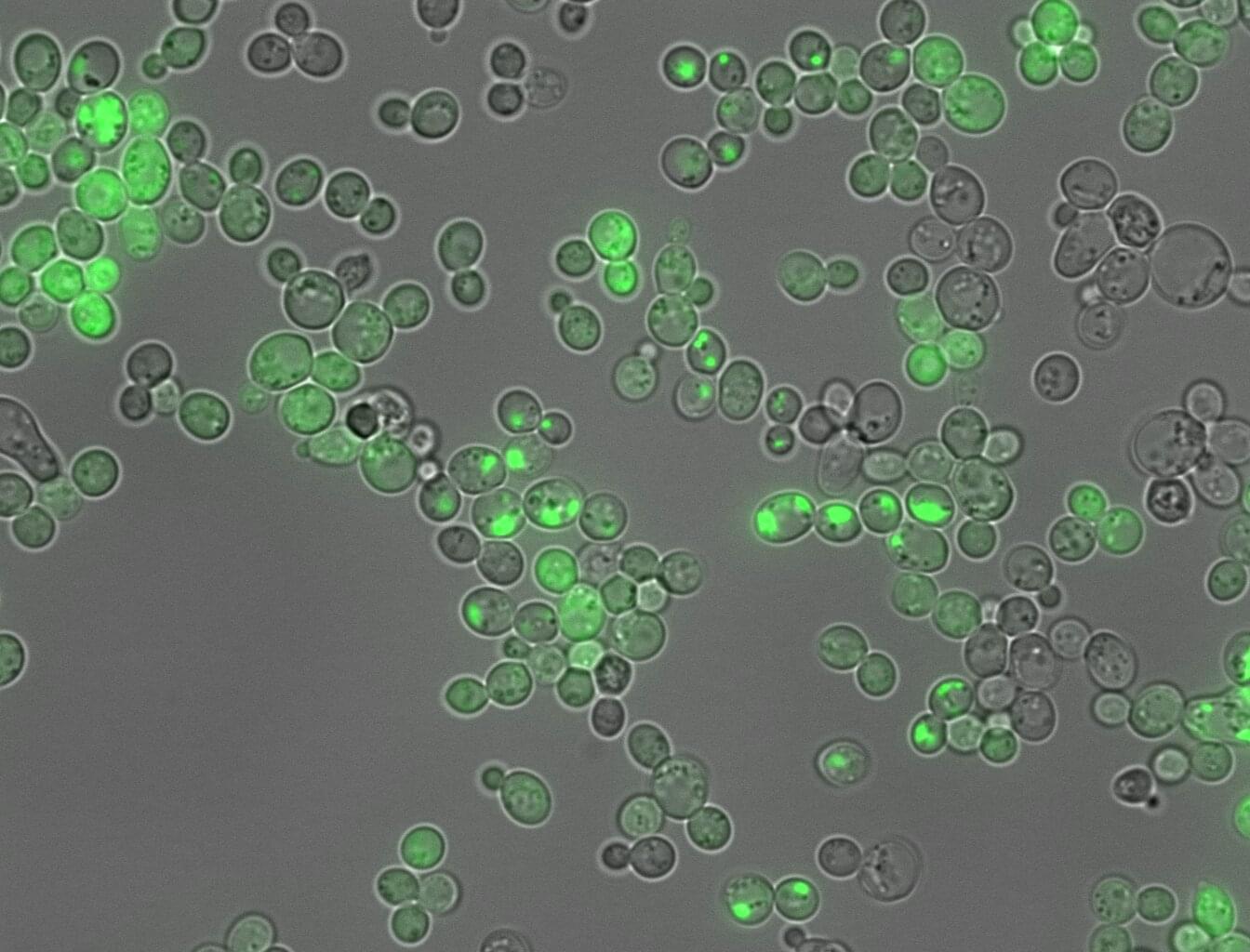
An AI tool has made a step forward in translating the language proteins use to dictate whether they form sticky clumps similar to those linked to Alzheimer’s disease and around fifty other types of human disease. In a departure from typical “black-box” AI models, the new tool, CANYA, was designed to be able to explain its decisions, revealing the specific chemical patterns that drive or prevent harmful protein folding.
The discovery, published in the journal Science Advances, was possible thanks to the largest-ever dataset on protein aggregation created to date. The study gives new insights about the molecular mechanisms underpinning sticky proteins, which are linked to diseases affecting half a billion people worldwide.
Protein clumping, or amyloid aggregation, is a health hazard that disrupts normal cell function. When certain patches in proteins stick to each other, proteins grow into dense fibrous masses that have pathological consequences.
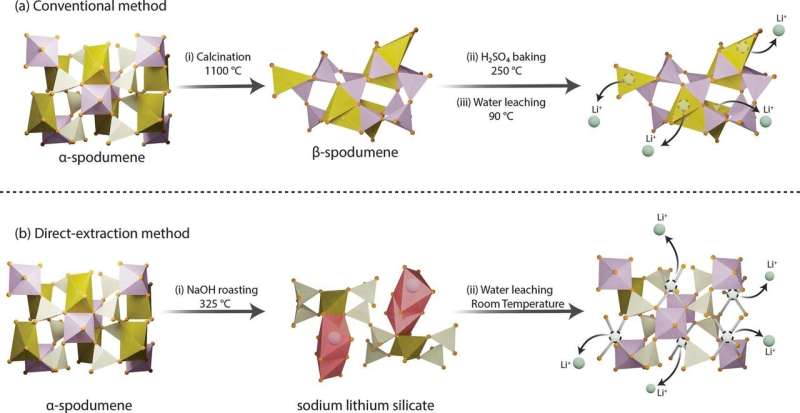
Lightweight lithium metal is a heavy-hitting critical mineral, serving as the key ingredient in the rechargeable batteries that power phones, laptops, electric vehicles and more. As ubiquitous as lithium is in modern technology, extracting the metal is complex and expensive. A new method, developed by researchers at Penn State and recently granted patent rights, enables high-efficiency lithium extraction—in minutes, not hours—using low temperatures and simple water-based leaching.
“Lithium powers the technologies that define our modern lives—from smartphones to electric vehicles—and has applications in grid energy storage, ceramics, glass, lubricants, and even medical and nuclear technologies,” said Mohammad Rezaee, the Centennial Career Development Professor in Mining Engineering at Penn State, who led the team that published their approach in Chemical Engineering Journal.
“But its extraction must also be environmentally responsible. Our research shows that we can extract lithium, and other critical minerals, more efficiently while drastically reducing energy use, greenhouse gas emissions and waste that’s difficult to manage or dispose of.”

A new study involving over 700 older adults suggests that taking one gram of omega-3 daily may help slow biological aging, with effects visible in molecular markers known as epigenetic clocks.
When combined with vitamin D and regular exercise, the anti-aging benefits became even more pronounced, lowering the risks of frailty and cancer as well.
Omega-3 linked to slower aging in humans.