A novel matrix could improve the consistency of stem cell cultures and boost production to industrial scales.



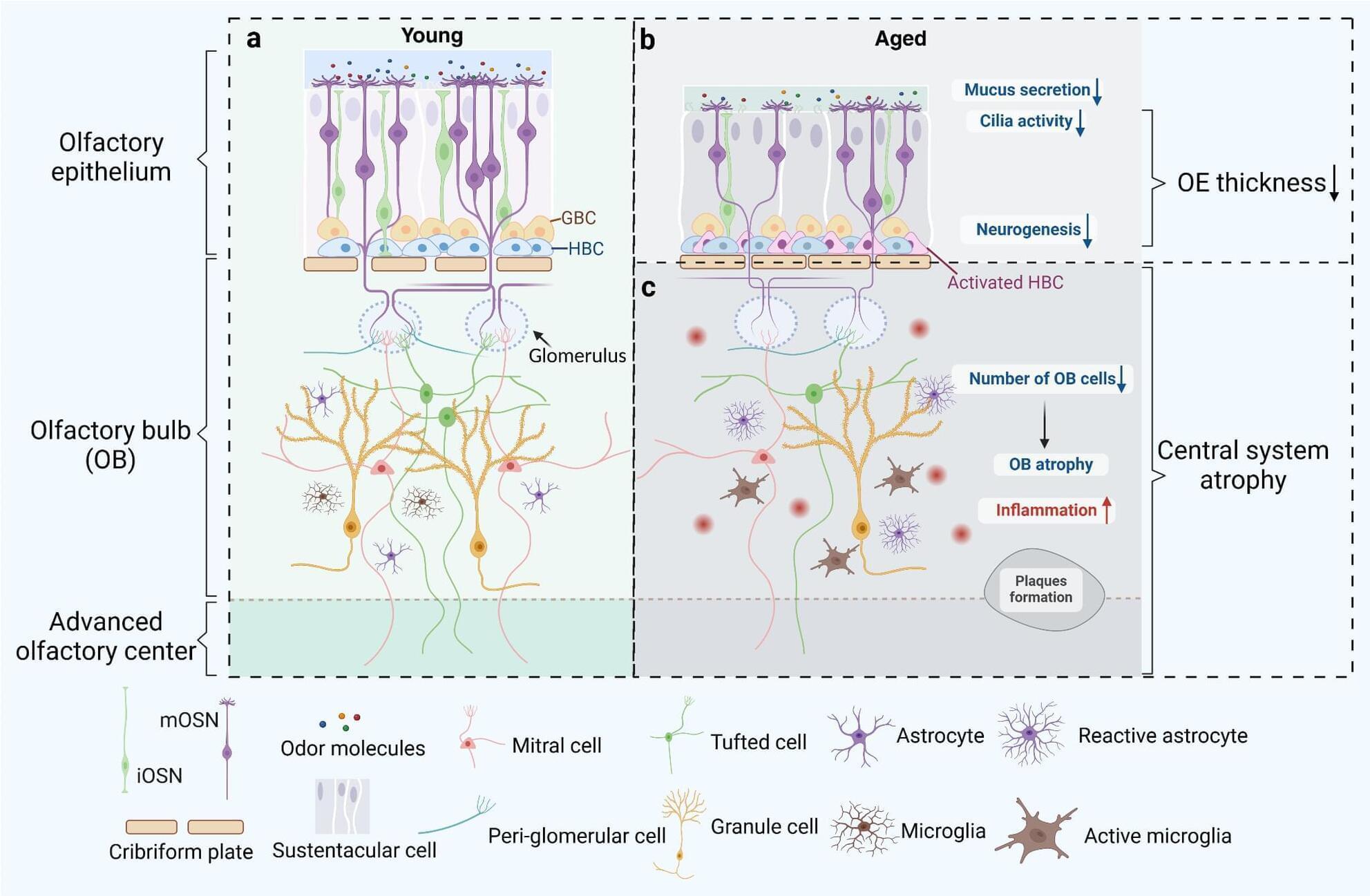
Olfactory dysfunction (OD) is a common nasal disease, particularly prevalent among the elderly population, significantly impacting the affected individuals’ quality of life. This review focuses on the influence of aging and chronic inflammation on olfactory dysfunction, presenting insights from both the peripheral and central olfactory systems. By exploring the molecular mechanisms and pathological changes underlying the occurrence of olfactory dysfunction in relation to age-related diseases and chronic inflammation conditions, we aim to provide a comprehensive theoretical foundation for further research and offer valuable insights for more effective treatment of olfactory dysfunction.
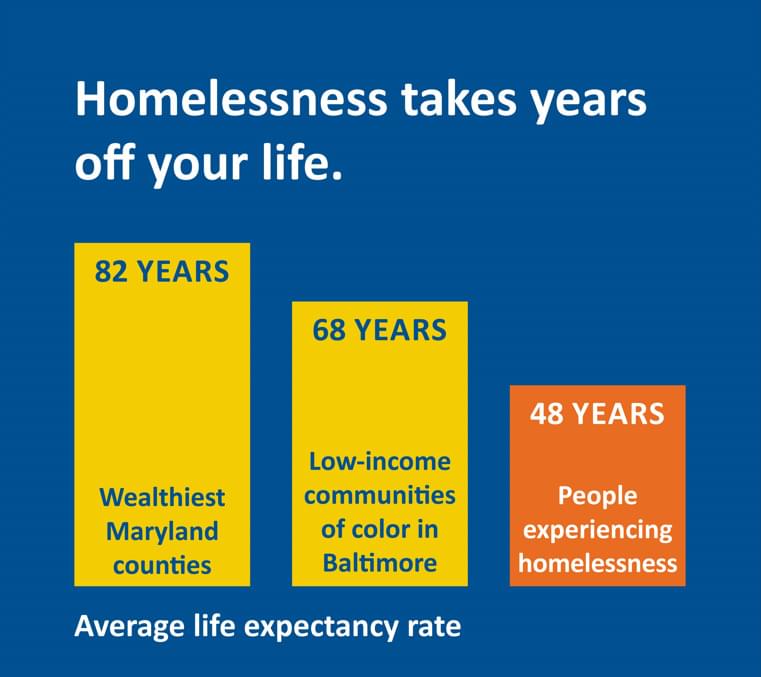


The risk of getting dementia may go up as you get older if you don’t get enough slow-wave sleep.
A 2023 study found that over-60s are 27 percent more likely to develop dementia if they lose just 1 percent of this deep sleep each year.
Slow-wave sleep is the third stage of a human 90-minute sleep cycle, lasting about 20–40 minutes. It’s the most restful stage, where brain waves and heart rate slow and blood pressure drops.
Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhD
Discount Links/Affiliates:
Blood testing (where I get the majority of my labs): https://www.ultalabtests.com/partners/michaellustgarten.
At-Home Metabolomics: https://www.iollo.com?ref=michael-lustgarten.
Use Code: CONQUERAGING At Checkout.
Clearly Filtered Water Filter: https://get.aspr.app/SHoPY
Epigenetic, Telomere Testing: https://trudiagnostic.com/?irclickid=U-s3Ii2r7xyIU-LSYLyQdQ6…M0&irgwc=1
Use Code: CONQUERAGING
NAD+ Quantification: https://www.jinfiniti.com/intracellular-nad-test/
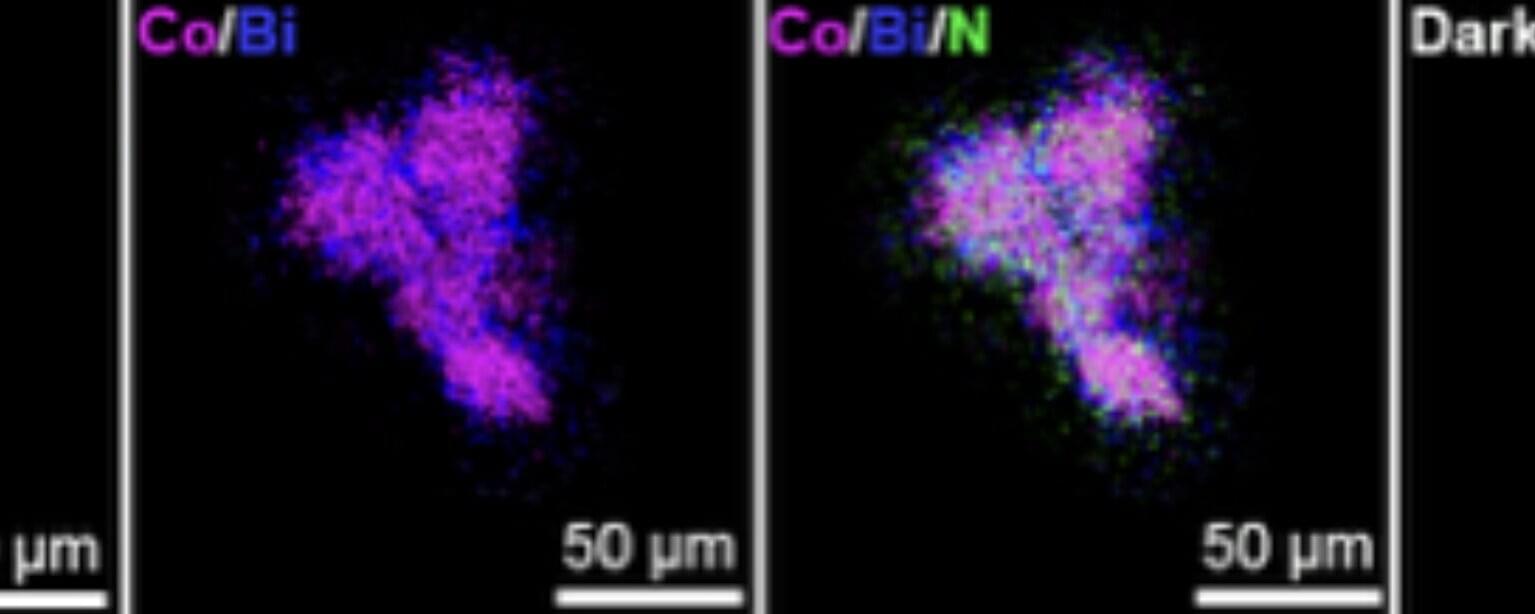
Recent technological advances are fueling the development of cutting-edge technologies that can monitor and control physiological processes with high precision. These include devices that could control the expression of genes within living organisms, without requiring invasive surgeries or procedures.
Researchers at ETH Zurich recently introduced a new method that enables the electromagnetic programming of the wireless expression regulation (EMPOWER) of transgenes in mammals, via the interfacing of nanoparticles and cells.
Their proposed approach, outlined in a paper published in Nature Nanotechnology, could help to treat chronic conditions, including diabetes, while also opening new possibilities for research in synthetic biology and regenerative medicine.

