They could someday help millions get their voices back.


Transplanted embryonic neurons can properly connect into the developed visual cortex of adult mice and improve the animals’ sensitivities to visual cues over time, scientists reported today (October 26) in Nature. By demonstrating that added neurons can become fully functional in circuits that normally do not rewire in adulthood, the team’s results suggest that the brain may be more plastic than previously thought.
“Calorie restriction has been known to extend lifespan for a long time.” said Dr. Kennedy. “The DNA damage response is linked to aging as well. LOS1 may be connecting these different processes.”
A number of the age-extending genes the team identified are also found in C. elegans roundworms, indicating these mechanisms are conserved in higher organisms. In fact, many of the anti-aging pathways associated with yeast genes are maintained all the way to humans.
The research produced another positive result: exposing emerging scientists to advanced lab techniques, many for the first time.
SRF Summer Scholars Program opens December 1st!
The SRF Summer Scholars Program offers undergraduate students the opportunity to conduct biomedical research to combat diseases of aging, such as cancer, Alzheimer’s, and Parkinson’s Disease. Under the guidance of a scientific mentor, each Summer Scholar is responsible for his or her own research project in such areas as genetic engineering and stem cell research. The Summer Scholars Program emphasizes development of both laboratory and communication skills to develop well-rounded future scientists, healthcare professionals, and policy makers. Students participating in the program will hone their writing skills via periodic reports, which are designed to emulate text scientists commonly must produce. At the end of the summer, students will have the opportunity to put all of their newly developed communication skills into practice at a student symposium.
Optalysys’s technology performs a mathematical function called the Fourier transform by encoding data, say a genome sequence, into a laser beam. The data can be manipulated by making light waves in the beam interfere with one another, performing the calculation by exploiting the physics of light, and generating a pattern that encodes the result. The pattern is read by a camera sensor and fed back into a conventional computer’s electronic circuits. The optical approach is faster because it achieves in a single step what would take many operations of an electronic computer.
The technology was enabled by the consumer electronics industry driving down the cost of components called spatial light modulators, which are used to control light inside projectors. The company plans to release its first product next year, aimed at high-performance computers used for processing genomic data. It will take the form of a PCI express card, a standard component used to upgrade PCs or servers usually used for graphics processors. Optalysys is also working on a Pentagon research project investigating technologies that might shrink supercomputers to desktop size, and a European project on improving weather simulations.
In 2015, Optalysis built a prototype that achieves a processing speed equivalent to 320 Gflops and it is incredibly energy efficient as it uses low-powered, cost effective components.

With the phone, predictions now feel relatively easy. But we’re setting off on our next five years, and we’re looking beyond the phone. What happens next? And what does it mean for how we live in the future? For our anniversary, we asked 10 of the smartest, most interesting, most influential people we know to describe our lives in 2021 — and the many ways technology, culture, science, and transportation will change. We’ll be running these interviews all through November, and they paint an ambitious, dynamic vision of the future.
We’ll discuss how in the near future, many Americans may never drive again. We’ll talk to groundbreaking scientists about CRISPR, a revolutionary method of editing genes that’s already led to incredible breakthroughs. We’ll see how for many employees, technology may make geography irrelevant, and how social media will usher in a new age of social activism. More women will finally find their rightful place in boardrooms, and by 2021, artificial and human intelligence will exist in something called “symbiotic autonomy.”
It’s tempting to look backwards on an anniversary. But The Verge is about looking ahead, and we would much rather spend our fifth birthday imagining the incredible (and occasionally terrifying) promise of the future. We’ve collected some excellent guides to help us along the way — we hope you join us.


In Brief:
Our life experiences may be passed on to our children and our children’s children — and now scientists report that they have discovered that this inheritance can be turned on or off.
Epigenetics is the study of inherited changes in gene expression…changes that are inherited, but they are not inherent to our DNA. For instance, life experiences, which aren’t directly coded in human DNA, can actually be passed on to children. Studies have shown that survivors of traumatic events may have effects in subsequent generations.

More progress with gene therapy safety.
A Washington State University researcher has developed a way to reduce the development of cancer cells that are an infrequent but dangerous byproduct of gene therapy.
Grant Trobridge, an associate professor of pharmaceutical sciences, has altered the way a virus carries a beneficial gene to its target cell. The modified viral vectors reduce the risk of cancer and can be used for many blood diseases.
Trobridge and his team report their development in Scientific Reports, an online open-access journal produced by the Nature Publishing Group. The team is translating their findings into a stem cell gene therapy to target a life-threatening immunodeficiency in newborns called SCID-X1, also known as “Boy in the Bubble Syndrome.”
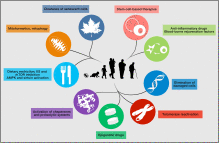
2013 saw the release of one of the most important papers in aging research and one that saw renewed interest and support for the concept of SENS.
Aging is characterized by a progressive loss of physiological integrity, leading to impaired function and increased vulnerability to death. This deterioration is the primary risk factor for major human pathologies, including cancer, diabetes, cardiovascular disorders, and neurodegenerative diseases. Aging research has experienced an unprecedented advance over recent years, particularly with the discovery that the rate of aging is controlled, at least to some extent, by genetic pathways and biochemical processes conserved in evolution. This Review enumerates nine tentative hallmarks that represent common denominators of aging in different organisms, with special emphasis on mammalian aging. These hallmarks are: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. A major challenge is to dissect the interconnectedness between the candidate hallmarks and their relative contributions to aging, with the final goal of identifying pharmaceutical targets to improve human health during aging, with minimal side effects.