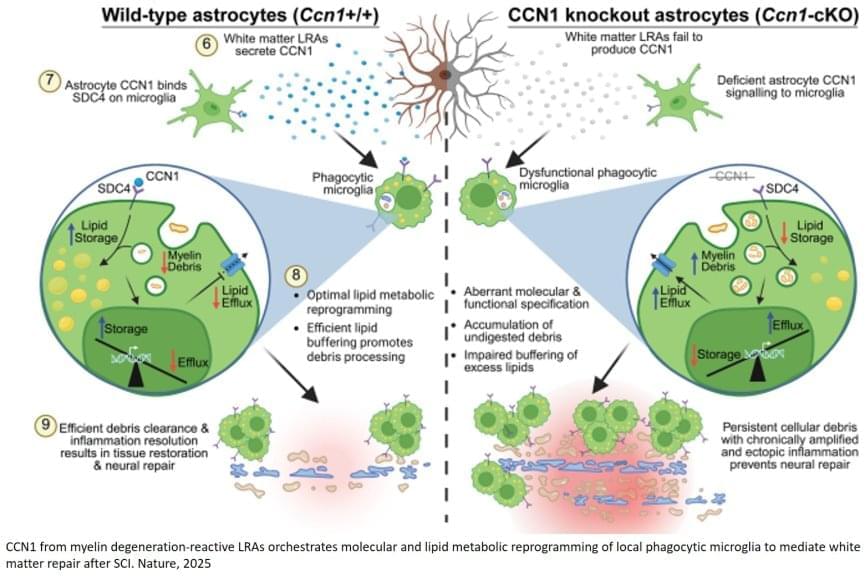
In acute ischemic stroke, insulin resistance worsens outcomes by increasing blood-brain barrier permeability in the ischemic core.
Acute large‐vessel occlusion of head and neck severely affect quality of life. One of the critical pathological events associated with prognosis of acute ischemic stroke (AIS) is the disruption of the blood–brain barrier (BBB), a highly selective barrier maintaining the brain’s microenvironment.1 Ischemia causes BBB dysfunction,2 exacerbated by peripheral immune cell infiltration,3 leading to increased parenchymal injury, hemorrhage,4 and edema.4, 5 Therefore, accurately determining the evolution and severity of BBB permeability are crucial for prognostic evaluation in patients with AIS.
Research on BBB disruption in patients with AIS primarily focuses on pathological mechanisms and imaging evaluations. Numerous studies use animal models and cell culture experiments to elucidate the physiological and molecular bases of BBB disruption.2, 5, 6, 7, 8 Additionally, imaging techniques like magnetic resonance imaging are widely used to assess evolution of BBB permeability1, 9, 10, 11, 12 and demonstrate correlations between BBB damage, cerebral edema, hemorrhagic transformation, and poor prognosis. Despite significant advances, several issues remain unresolved. First, differences between animal models and human disease limit clinical applicability. Second, while magnetic resonance imaging is widely used to provide quantitative data on BBB disruption, they still face the limitations of being time consuming and inconvenient in the clinical environment. However, BBB disruption in patients with AIS based on computed tomography perfusion (CTP) needs further study.
Insulin resistance (IR) is linked to adverse cardiovascular13, 14 and metabolic outcomes.15, 16 Therefore, identifying patients with IR aids early risk stratification and management. The triglyceride–glucose (TyG) index, which combines fasting triglyceride and glucose levels, has been proposed as a marker of IR. Currently, research on the TyG index primarily focuses on type 2 diabetes,17 obesity,18 and cardiovascular diseases.19 Recent studies have found that an elevated TyG index is associated with higher stroke recurrence,19, 20 functional deterioration,21, 22 and death.23 However, whether the underlying mechanisms involved in the association of IR with stroke outcomes have not been fully understood. IR exacerbated vascular inflammation and endothelial dysfunction,24, 25, 26 which were critical contributors to BBB disruption in AIS. Previous studies have shown that metabolic dysregulation, including hypertriglyceridemia27 and hyperglycemia,28 aggravates BBB permeability by triggering oxidative stress and inflammatory pathways.29, 30 Specifically, IR leads to the generation of reactive oxygen species and the activation of NADPH oxidase, which contribute to oxidative stress and endothelial damage.30, 31 Inflammatory cytokines such as tumor necrosis factor‐α, interleukin‐1β, and interleukin‐6 are also elevated in the insulin‐resistant state, activating nuclear factor‐κB and Janus kinase signaling pathways that further compromise endothelial tight junction proteins such as occludin, claudin‐5, and zonula occludens‐1, crucial for BBB integrity.29, 32 These mechanisms are known to increase BBB permeability, facilitating the entry of harmful substances into the brain and contributing to worsened stroke outcomes. These findings indicated that the relationship between IR and stroke outcomes may be mediated by increased BBB permeability. However, the TyG index’s relationship with BBB disruption and outcomes in patients with AIS is not well studied.