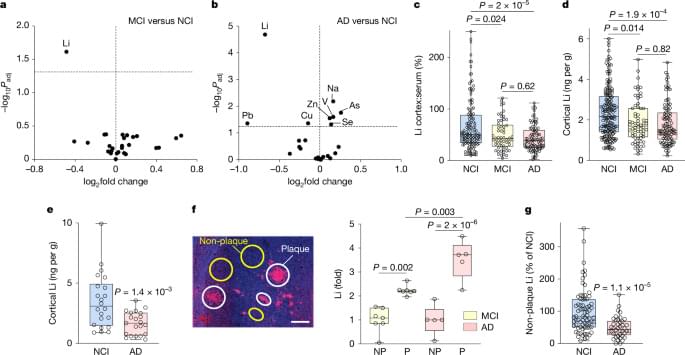
Lithium has an essential role in the brain and is deficient early in Alzheimer’s disease, which can be recapitulated in mice and treated with a novel lithium salt that restores the physiological level.


As a chronic condition, rheumatoid arthritis (RA) can’t be cured, so treatment focuses on managing the disease and controlling its progression. Although current treatments help control RA symptoms in most people, they cannot prevent the onset of RA or painful flare-ups.
Now, researchers publishing in ACS Central Science have developed nanoparticles that could slow disease progression and reduce flare severity, based on results from tests with human blood and mice models with RA-like disease.
For a person diagnosed with RA, their immune system attacks tissue that makes up the joints, causing inflammation, swelling and pain. However, as the disease progresses, serious cartilage and bone damage can occur if left uncontrolled.

A study published in Cell Reports Medicine reports a scalable, data-driven computational framework for designing combinatorial immunotherapies, offering hope for patients with poor responses to current immunotherapies.
Immunotherapy, particularly immune checkpoint blockade (ICB), has revolutionized cancer treatment. Widespread resistance to ICB is a major challenge in clinical practice.
To enhance treatment efficacy and overcome resistance, combining ICB therapy with chemotherapy or targeted therapy has become an important research direction. However, candidate combinations rely on empirical selection from existing drugs, and it is difficult to discover new candidates.

Eating pistachios every night for 12 weeks altered bacteria in the gut, according to new study. A new study reveals that swapping a typical nighttime carbohydrate snack for pistachios may beneficially alter gut bacteria in people with prediabetes. Conducted by Penn State researchers, the 12-week clinical trial found that pistachio consumption increased beneficial gut microbes like Roseburia and reduced harmful ones such as Blautia hydrogenotrophica. These microbiome changes could potentially support metabolic health and slow the progression to Type 2 diabetes. While more research is needed to confirm health outcomes, this study positions pistachios as a promising late-night snack with microbiome-boosting potential.
Prediabetes affects a third of people in the United States and most of them will develop Type 2 diabetes, yet effective dietary intervention strategies remain limited. Pistachios have shown promise in improving markers of diet quality, yet little is known about how they influence the gut microbiome — a key player in glucose regulation and inflammation.
A new study led by Kristina Petersen, associate professor of nutritional sciences at Penn State, determined that nighttime pistachio consumption affects gut bacteria in adults with prediabetes. Though the potential therapeutic implications of the findings remain unclear, according to Petersen, they may prove significant for people who are working to improve their metabolic health.
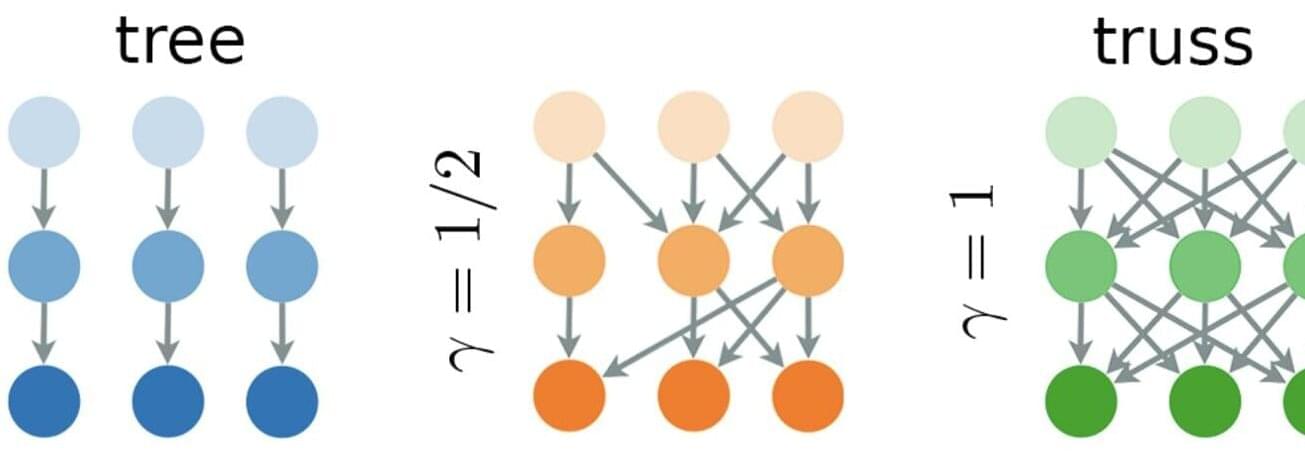
Research shows that while connections between innovations speed discovery, they also sharply increase the risk of total system collapse—with the sweet spot for sustainable innovation proving surprisingly narrow.
Innovation is a central currency of global power. Whether in the race for leadership in artificial intelligence, the development of clean energy technologies, or the search for medical breakthroughs, major players like China, the United States, and the European Union are investing billions in research and development to secure the next technological leap—and with it, economic and strategic advantage.
Yet, as a new study from the Complexity Science Hub (CSH), published in Physical Review Research, indicates, long-term innovation is only sustainable under specific structural conditions. First, the study finds that innovation can only endure over time if it is balanced with “exnovation”—the loss or forgetting of older possibilities.
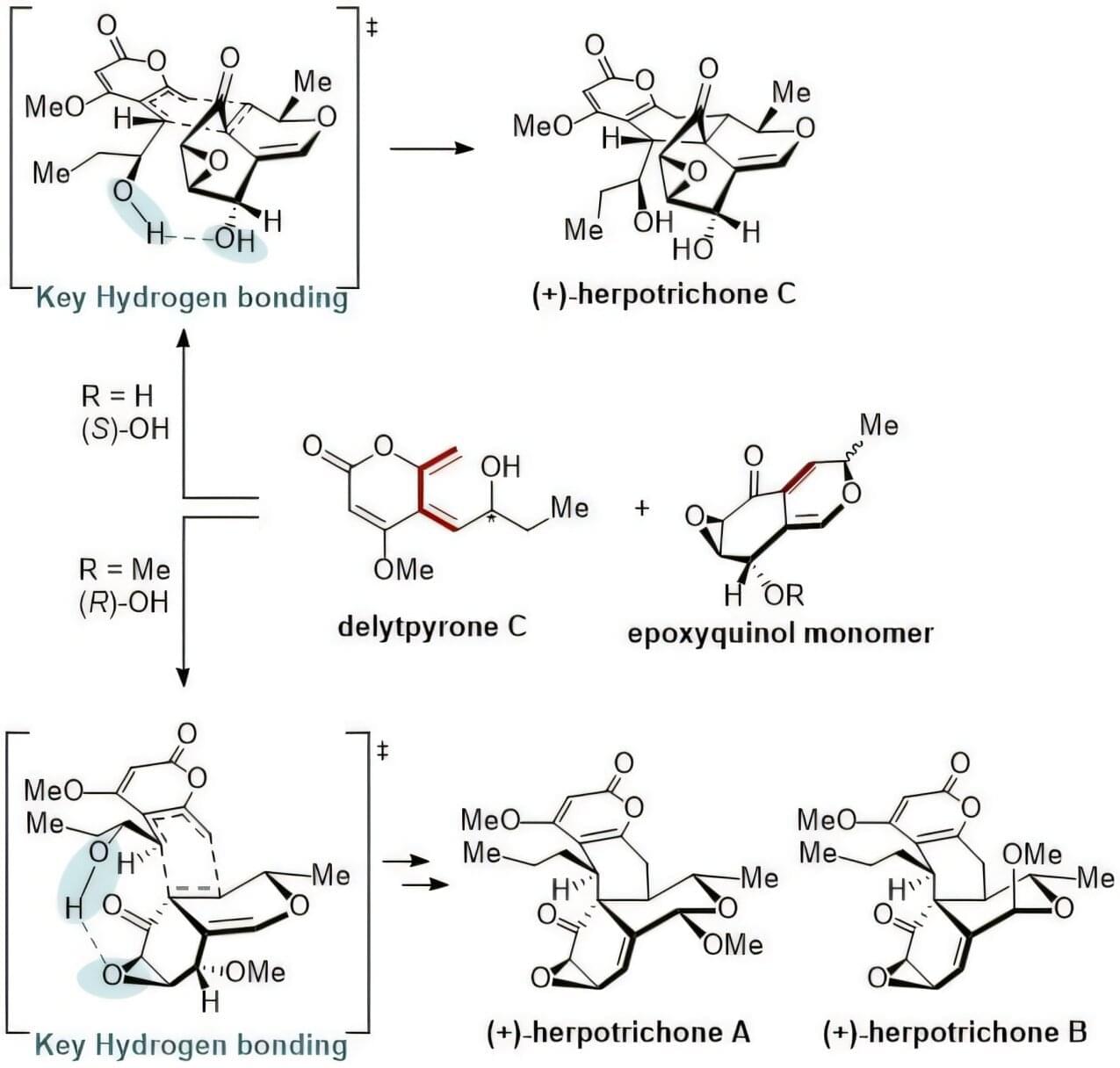
“Herpotrichone” is a natural substance that has been evaluated highly for its excellent ability to suppress inflammation in the brain and protect nerve cells, displaying significant potential to be developed as a therapeutic agent for neurodegenerative brain diseases such as Alzheimer’s disease and Parkinson’s disease. This substance could only be obtained in minute quantities from fungi that are symbiotic with isopods. However, KAIST researchers have succeeded in chemically synthesizing this rare natural product, thereby presenting the possibility for the development of next-generation drugs for neurodegenerative diseases.
A research team led by Professor Sunkyu Han of the Department of Chemistry successfully synthesized the natural anti-neuroinflammatory substances ‘herpotrichones A, B, and C’ for the first time. The paper is published in the Journal of the American Chemical Society.
Herpotrichone natural products are substances obtainable only in minute quantities from Herpotrichia sp. SF09, a symbiotic pill bug fungus, and possesses a unique 6÷6÷6÷6÷3 pentacyclic framework consisting of five fused rings (four six-membered and one three-membered ring).

Marine cone snails are host to a family of dangerous neurotoxins. Very little is known about how those toxins interact with the human body, making this an area of interest for medical drug research and an area of concern in national security spaces. For the first time, a team at Los Alamos National Laboratory has successfully trained a machine learning model that predicts how alpha conotoxins bind to specific human receptor subtypes, which could help researchers develop lifesaving anti-toxins.
“Because of the diversity and complexity of natural conotoxins, it is estimated that only 2% of them have been sequenced,” said Gnana Gnanakaran, theoretical biologist at Los Alamos. “No antidotes exist for conotoxins, but by using machine learning to predict conotoxin binding, we now have the ability to develop tools to understand and respond to these threats.”
The deadly secretions issued by any one of the more than 800 cone snail species represent a conglomeration of more than 1 million natural conotoxins. The research team concentrated their machine learning work on alpha conotoxins, a particularly prevalent and deadly conotoxin family.

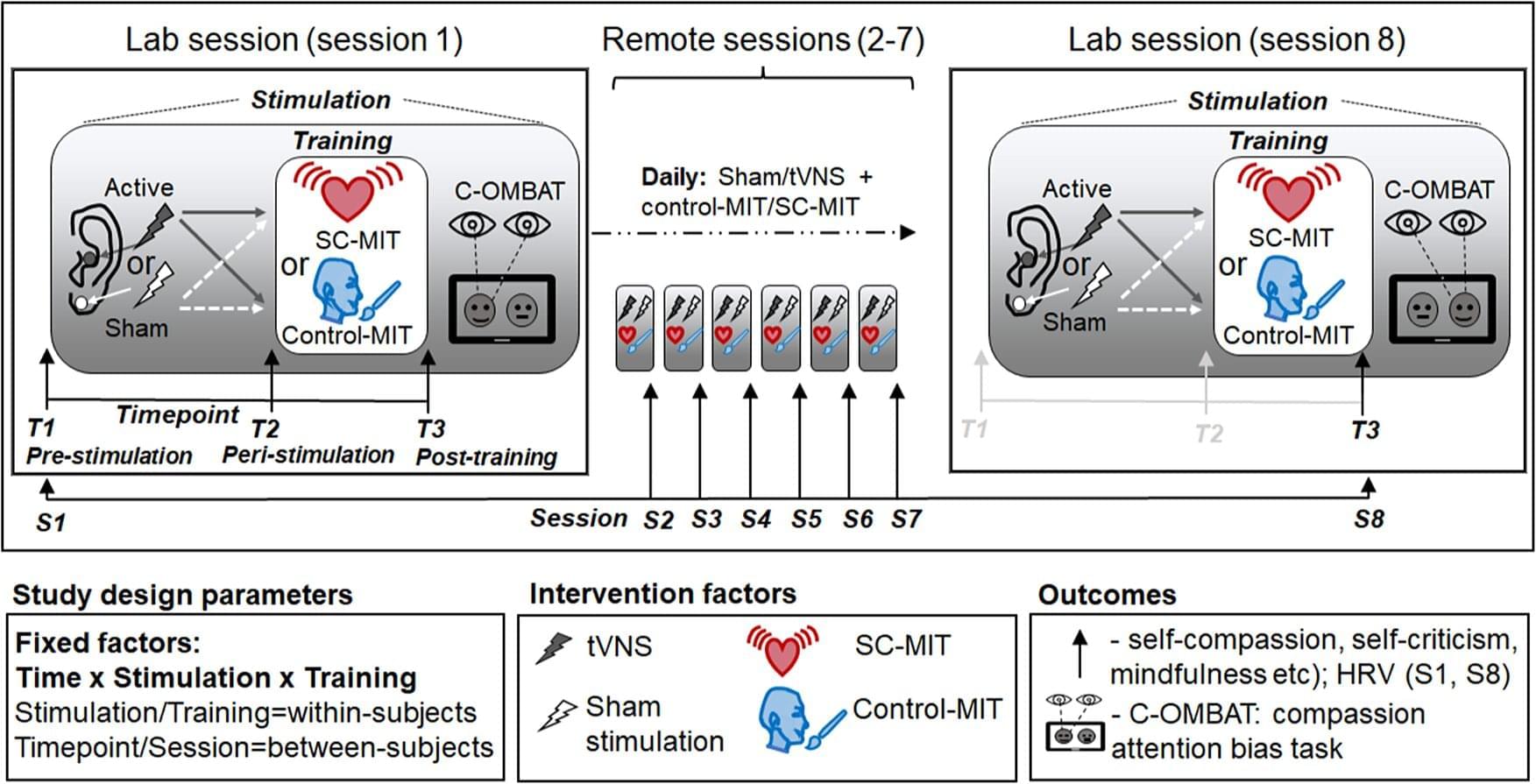
Stimulating the vagus nerve with a device attached to the outer ear can help make compassion meditation training more effective at boosting people’s capacity for self-kindness and mindfulness, finds a new study led by University College London (UCL) researchers.
The study, published in Psychological Medicine, adds to evidence of the potential benefits of stimulating this key nerve that connects the brain with major organs in the chest and abdomen.
The vagus nerve plays a crucial role in the “rest-and-digest” (parasympathetic) system, counteracting the “fight-or-flight” (sympathetic) stress response, and allows the brain to communicate with all major organs in the body. By transmitting signals from the body up to the brain, the vagus nerve can also regulate a range of psychological processes, including some involved in social interactions and emotional control.