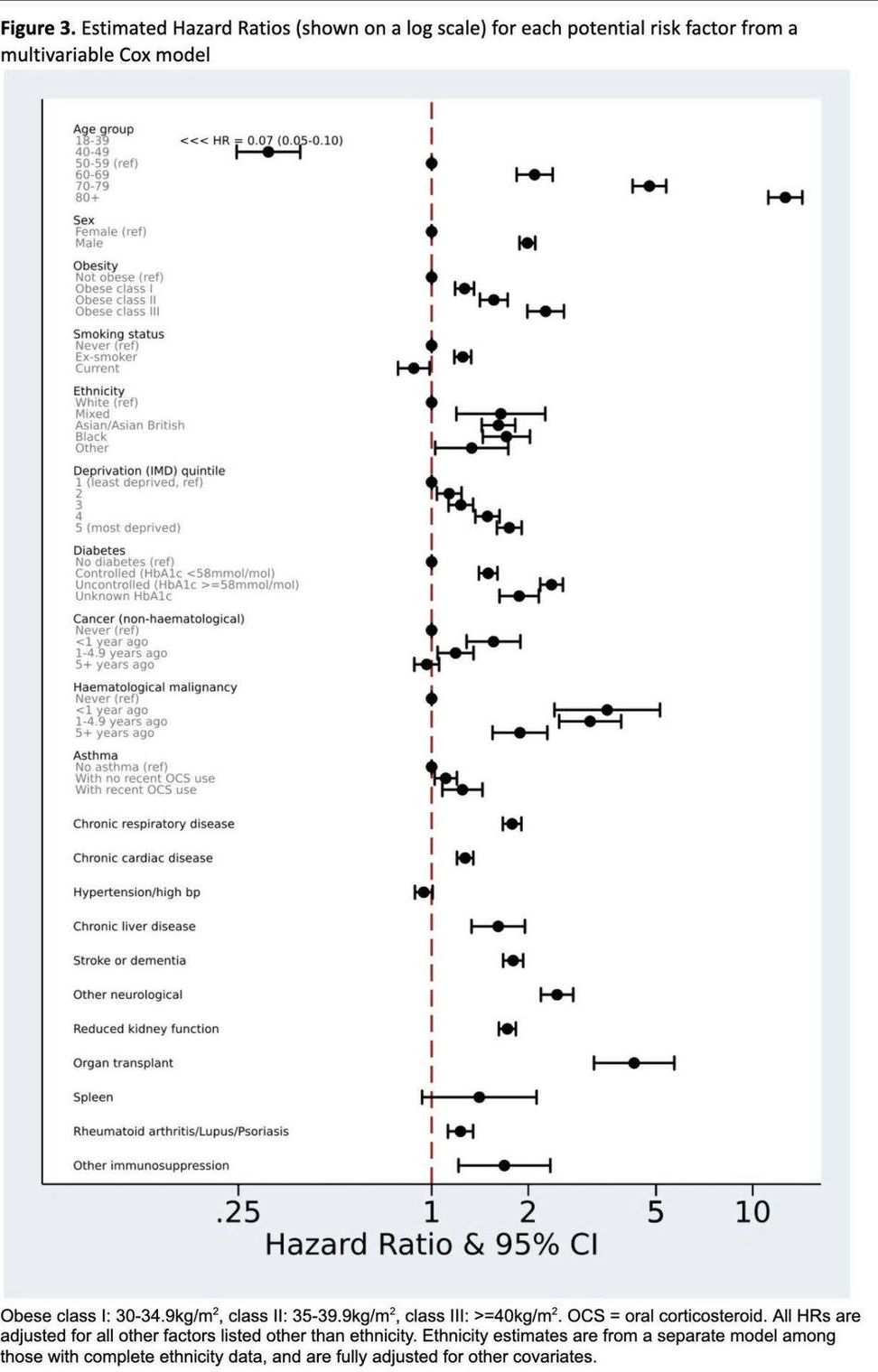
Scientists have discovered a new treatment to dramatically reduce swelling after brain and spinal cord injuries, offering hope to 75 million victims worldwide each year.
The breakthrough in treating such injuries—referred to as central nervous system (CNS) edema—is thought to be hugely significant because current options are limited to putting patients in an induced coma or performing risky surgery.
Brain and spinal cord injuries affect all age groups. Older people are more at risk of sustaining them from strokes or falls, while for younger age groups, major causes include road traffic accidents and injuries from sports such as rugby, US-style football and other contact games.