There’s a deluge of apps that detect your covid-19 exposure, often with little transparency. Our Covid Tracing Tracker project will document them.
By

There’s a deluge of apps that detect your covid-19 exposure, often with little transparency. Our Covid Tracing Tracker project will document them.
By

The cocktail, now named REGN-COV2, consists of two antibodies—REGN10933 and REGN10987—that are designed to bind non-competitively to the receptor binding domain of SARS-CoV-2’s spike protein. Regeneron says that such binding diminishes the ability of mutant viruses to escape treatment—with details from preclinical research to be published in upcoming research studies.
Regeneron Pharmaceuticals said today it has launched the first clinical trial of its dual-antibody “cocktail” designed to both prevent and treat COVID-19, as well as prevent viral escape. The cocktail, now named REGN-COV2, consists of two antibodies—REGN10933 and REGN10987—that are designed to bind non-competitively to the receptor binding domain of SARS-CoV-2’s spike protein. [Regeneron].

CLEW, an Israeli medtech firm specializing in real-time AI analytics platforms, received approval from the United States Food and Drug Administration (FDA) for its “Predictive Analytics Platform in Support of COVID-19 Patients,” the company announced Tuesday.
The Intensive Care Unit (ICU) solution was given Emergency Use Authorization (EUA) by the FDA so that it may be implemented within the United States’ health system as soon as possible.


A human embryo editing experiment gone wrong has scientists warning against treading into the field altogether.
To understand the role of a single gene in early human development, a team of scientists at the London-based Francis Crick Institute removed it from a set of 18 donated embryos. Even though the embryos were destroyed after just 14 days, that was enough time for the single edit to transform into “major unintended edits,” OneZero reports.
Human gene editing is a taboo topic — the birth of two genetically modified babies in 2018 proved incredibly controversial, and editing embryos beyond experimentation is not allowed in the U.S. The scientists in London conducted short-term research on a set of 25 donated embryos, using the CRISPR technique to remove a gene from 18 of them. An analysis later revealed 10 of those edited embryos looked normal, but that the other eight revealed “abnormalities across a particular chromosome,” OneZero writes. Of them, “four contained inadvertent deletions or additions of DNA directly adjacent to the edited gene,” OneZero continues.

Back in 2005, Drs. Irina and Michael Conboy showed that joining the circulatory systems of young and old mice together in a procedure called parabiosis could rejuvenate aged tissues and reverse some aspects of aging in old mice.
Following this discovery, many researchers concluded that there must be something special in young blood that was able to spur rejuvenation in aged animals, and various companies have been trying to find out what. Indeed, we recently reported that researchers were apparently successful in halving the epigenetic age of old rats by treating them with Elixir, a proprietary mix of pro-youthful factors normally found in young blood.
However, a question still remains: was the rejuvenation the result of there being something beneficial in the young blood, or is it more a case of dilution of the harmful factors present in old blood?
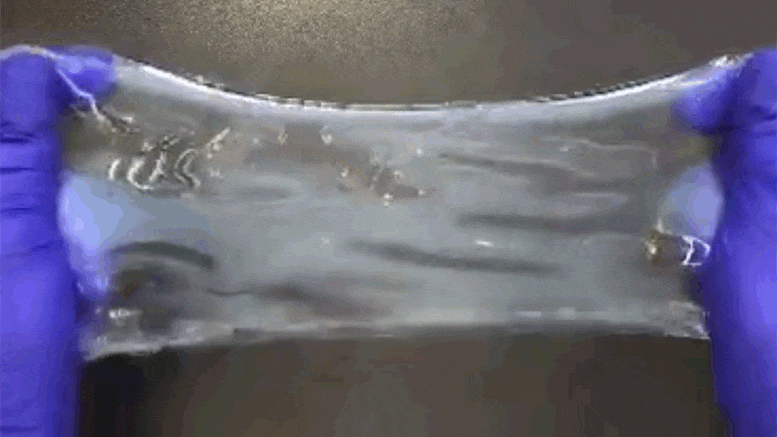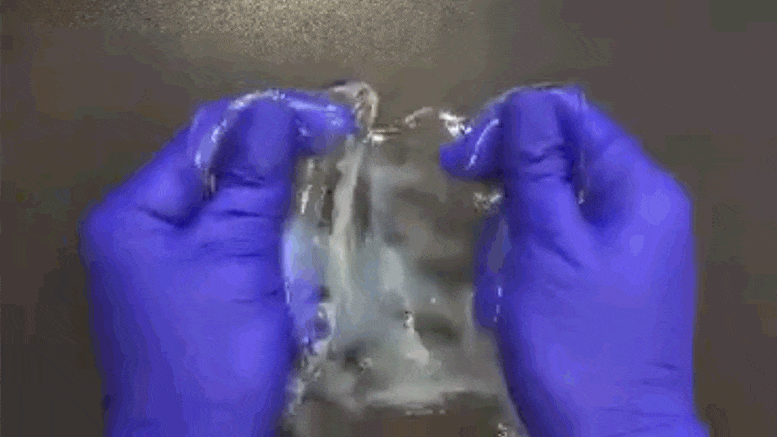
Chemical process called ELAST allows labeling probes to infuse more quickly, and makes samples tough enough for repeated handling.
When there’s a vexing problem to be solved, people sometimes offer metaphorical advice such as “stretching the mind” or engaging in “flexible” thinking, but in confronting a problem facing many biomedical research labs, a team of MIT researchers has engineered a solution that is much more literal. To make imaging cells and molecules in brain and other large tissues easier while also making samples tough enough for years of handling in the lab, they have come up with a chemical process that makes tissue stretchable, compressible, and pretty much indestructible.
“ELAST” technology, described in a new paper in Nature Methods, provides scientists a very fast way to fluorescently label cells, proteins, genetic material, and other molecules within brains, kidneys, lungs, hearts, and other organs. That’s because when such tissues can be stretched out or squished down thin, labeling probes can infuse them far more rapidly. Several demonstrations in the paper show that even after repeated expansions or compressions to speed up labeling, tissues snap back to their original form unaltered except for the new labels.

Researchers from the University of Bath have developed motion capture technology that enables you to digitize your dog without a motion capture suit and using only one camera.
The software could be used for a wide range of purposes, from helping vets diagnose lameness and monitoring recovery of their canine patients, to entertainment applications such as making it easier to put digital representations of dogs into movies and video games.
Motion capture technology is widely used in the entertainment industry, where actors wear a suit dotted with white markers which are then precisely tracked in 3D space by multiple cameras taking images from different angles. Movement data can then be transferred onto a digital character for use in films or computer games.
A research team at Hadassah-University Medical Center in Jerusalem’s Ein Kerem has discovered what they believe causes coronavirus patients to become seriously ill and even die. They also say they have a way to treat the cause before it’s too late.
At least 30% of patients with coronavirus develop blood clots that block the flow of blood to their kidneys, heart and brain, as well as the lungs, according to international research.
Hadassah researchers discovered that the patients who form these fatal clots have an increased level of alpha defensin protein in their blood, explained Dr. Abd Alrauf Higavi, who directs a lab at Hadassah and has been studying blood clots for 30 years.
“Patients with mild symptoms have a low concentration of alpha defensin,” he said. “Patients with strong disease symptoms have high levels. The people who die have very high levels.”
The Hadassah team studied more than 700 blood samples from 80 patients who were admitted to the medical center during the first peak of the coronavirus outbreak in Israel. The results show that alpha defensin speeds up blood clot formation, which can cause pulmonary embolism, heart attacks and stroke. In addition, when blood clots form in the alveoli, whose function it is to exchange oxygen and carbon dioxide molecules to and from the bloodstream, this can lead to respiratory distress and eventually intubation.
Multiple studies have shown that around 80% of coronavirus patients who are intubated have died.