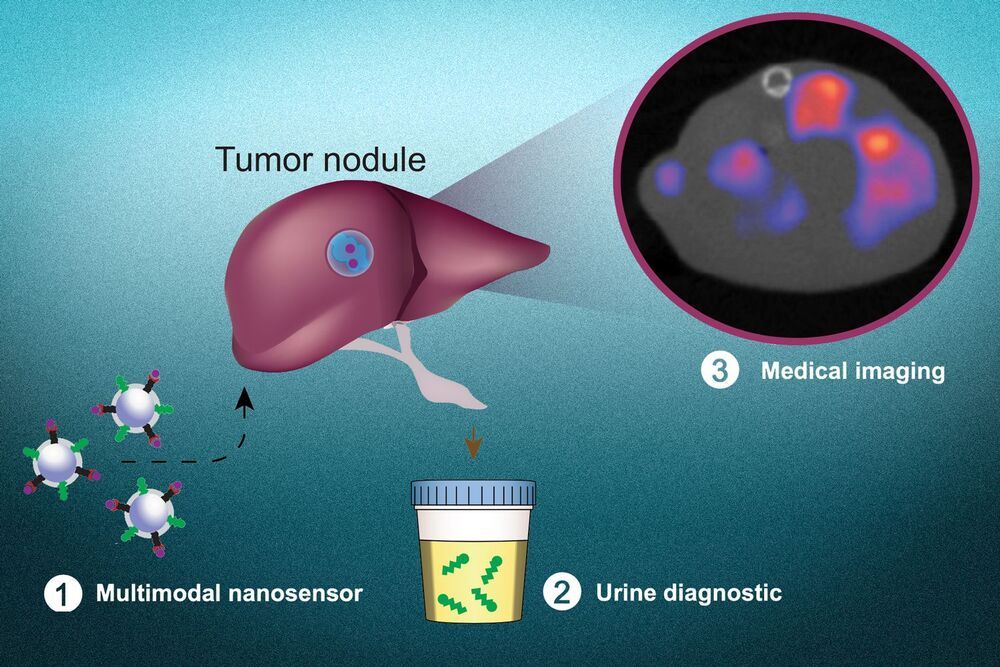
Health Innovation Investment For The Future Generations — Dr. Aboubacar Kampo, MD, MPH — Director of Health Programs — UNICEF.
Dr. Aboubacar Kampo, MD, MPH is the Director of Health Programs at UNICEF (UN Headquarters) where he provides strategic leadership, management support and overall direction to UNICEF’s global health program.
UNICEF, also known as the United Nations Children’s Emergency Fund, is a United Nations agency responsible for providing humanitarian and developmental aid to children worldwide. The agency is among the most widespread and recognizable social welfare organizations in the world, with a presence in 192 countries and territories. UNICEF’s activities include providing immunizations and disease prevention, administering treatment for children and mothers with HIV, enhancing childhood and maternal nutrition, improving sanitation, promoting education, and providing emergency relief in response to disasters.
With over 20 years of experience in development aid and humanitarian assistance, Dr. Kampo has worked as a physician/surgeon in hospitals and clinics in rural and urban areas in Africa and Asia and has over 14 years’ experience in senior management position as Country Director, Senior Global Health Advisor, and Chief of Health and Nutrition with International NGOs and United Nations’ Agencies.
Dr. Kampo is a Medical Doctor and Public Health Specialist, passionate about using innovations to address real life community challenges and bridge the gap between communities and stakeholders.