When a key protein regulator dials down DNA repair mechanisms, our cells accumulate more mutations, which may cause us to age faster


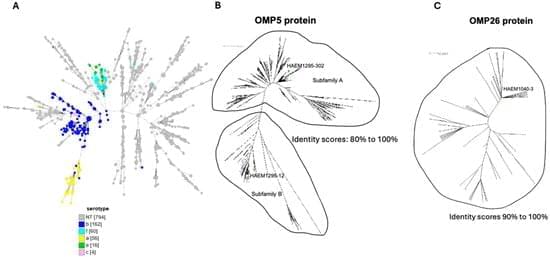
Background: Haemophilus influenzae (Hi), a Gram-negative bacterium, is divided into two broad categories: encapsulated and non-capsulated isolates, also called non-typeable Hi isolates (NTHi). NTHi has become prevalent since the introduction of the vaccine against Hi of serotype b. Hi can cause local infections on respiratory mucosal surfaces and urogenital infections, which can lead to septic abortion in pregnant women. It can also cause invasive infections such as meningitis and septicemia. Moreover, NTHi isolates are becoming increasingly resistant to antibiotics. Vaccines targeting NTHi are not yet available. As these NTHi isolates are not encapsulated, vaccines should target proteins at the bacterial surface. However, vaccine development is hindered by the high variability of these proteins. We aimed to identify conserved outer membrane proteins (OMPs) for vaccines against NTHi.
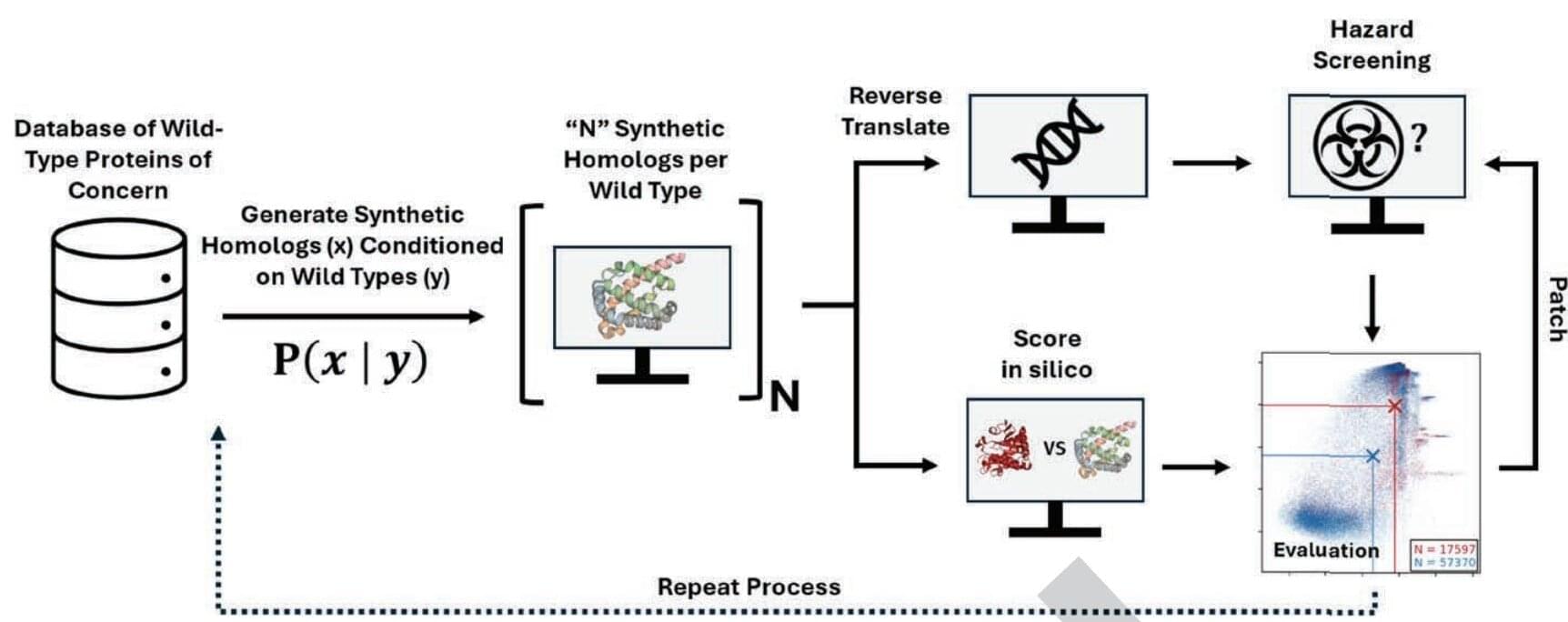
Artificial intelligence is transforming biology and medicine by accelerating the discovery of new drugs and proteins and making it easier to design and manipulate DNA, the building blocks of life. But as with most new technologies, there is a potential downside. The same AI tools could be used to develop dangerous new pathogens and toxins that bypass current security checks. In a new study from Microsoft, scientists employed a hacker-style test to demonstrate that AI-generated sequences could evade security software used by DNA manufacturers.
“We believe that the ongoing advancement of AI-assisted protein design holds great promise for tackling critical challenges in health and the life sciences, with the potential to deliver overwhelmingly positive impacts on people and society,” commented the researchers in their paper published in the journal Science. “As with other emerging technologies, however, it is also crucial to proactively identify and mitigate risks arising from novel capabilities.”
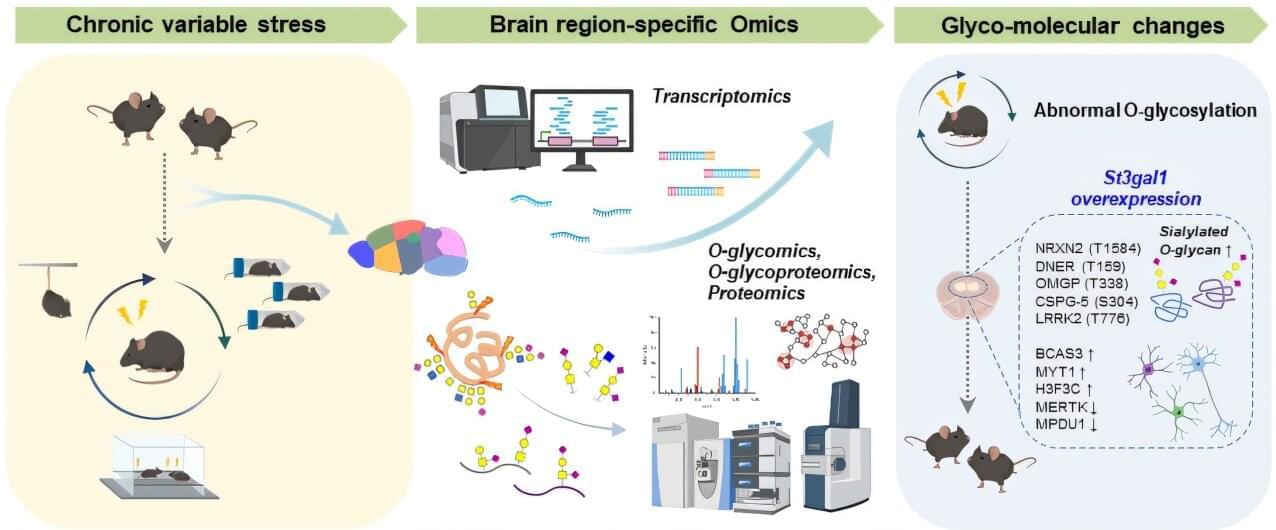
Depression is a serious disorder that disrupts daily life through lethargy, sleep disturbance, and social withdrawal, and also increases the risk of suicide. The number of depression patients has steadily increased over the years, affecting more than 280 million people worldwide as of 2025. Now, researchers have uncovered a new pathological mechanism that could provide clues for the diagnosis and treatment of depression.
A research team led by C. Justin Lee and Lee Boyoung at the Institute for Basic Science (IBS) has identified a new molecular pathway in the brain that directly links abnormal sugar modifications in proteins to depressive behaviors. Specifically, chronic stress disrupts sugar chains (O-glycans) attached to proteins in the prefrontal cortex, thereby triggering depression.
The findings, published in Science Advances, open new possibilities for targeted therapies for treatment-resistant depression.
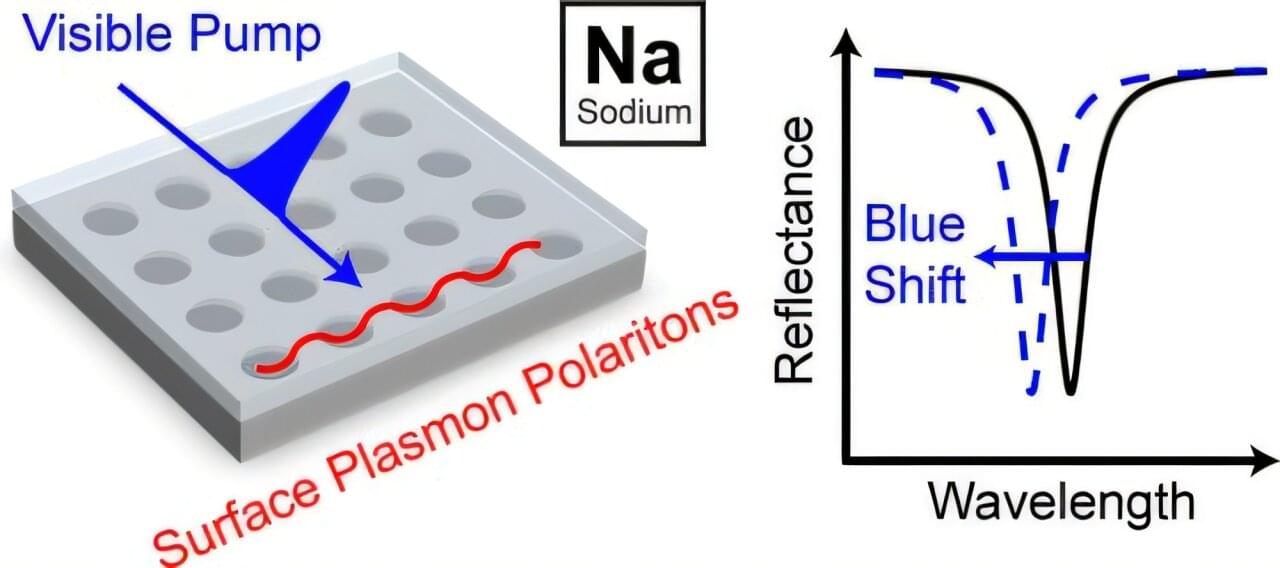
From solar panels to next-generation medical devices, many emerging technologies rely on materials that can manipulate light with extreme precision. These materials—called plasmonic materials—are typically made from expensive metals like gold or silver. But what if a cheaper, more abundant metal could do the job just as well or better?
That’s the question a team of researchers set out to explore. The challenge? While sodium is abundant and lightweight, it’s also notoriously unstable and difficult to work with in the presence of air or moisture—two unavoidable parts of real-world conditions. Until now, this has kept it off the table for practical optical applications.
Researchers from Yale University, Oakland University, and Cornell University have teamed up to change that. By developing a new technique for structuring sodium into ultra-thin, precisely patterned films, they found a way to stabilize the metal and make it perform exceptionally well in light-based applications.
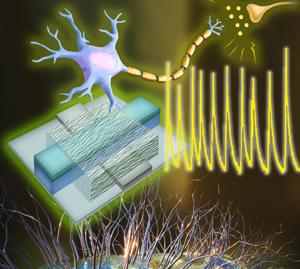
A team of engineers at the University of Massachusetts Amherst has announced the creation of an artificial neuron with electrical functions that closely mirror those of biological ones. Building on their previous groundbreaking work using protein nanowires synthesized from electricity-generating bacteria, the team’s discovery means that we could see immensely efficient computers built on biological principles which could interface directly with living cells.
“Our brain processes an enormous amount of data,” says Shuai Fu, a graduate student in electrical and computer engineering at UMass Amherst and lead author of the study published in Nature Communications. “But its power usage is very, very low, especially compared to the amount of electricity it takes to run a Large Language Model, like ChatGPT.”
The human body is over 100 times more electrically efficient than a computer’s electrical circuit. The human brain is composed of billions of neurons, specialized cells that send and receive electrical impulses all over the body. While it takes only about 20 watts for your brain to, say, write a story, a LLM might consume well over a megawatt of electricity to do the same task.
A team of engineers at the University of Massachusetts Amherst has announced the creation of an artificial neuron with electrical functions that closely mirror those of biological ones. Building on their previous groundbreaking work using protein nanowires synthesized from electricity-generating bacteria, the team’s discovery means that we could see immensely efficient computers built on biological principles which could interface directly with living cells.
“Our brain processes an enormous amount of data,” says Shuai Fu, a graduate student in electrical and computer engineering at UMass Amherst and lead author of the study published in Nature Communications. “But its power usage is very, very low, especially compared to the amount of electricity it takes to run a Large Language Model, like ChatGPT.”
The human body is over 100 times more electrically efficient than a computer’s electrical circuit. The human brain is composed of billions of neurons, specialized cells that send and receive electrical impulses all over the body. While it takes only about 20 watts for your brain to, say, write a story, a LLM might consume well over a megawatt of electricity to do the same task.
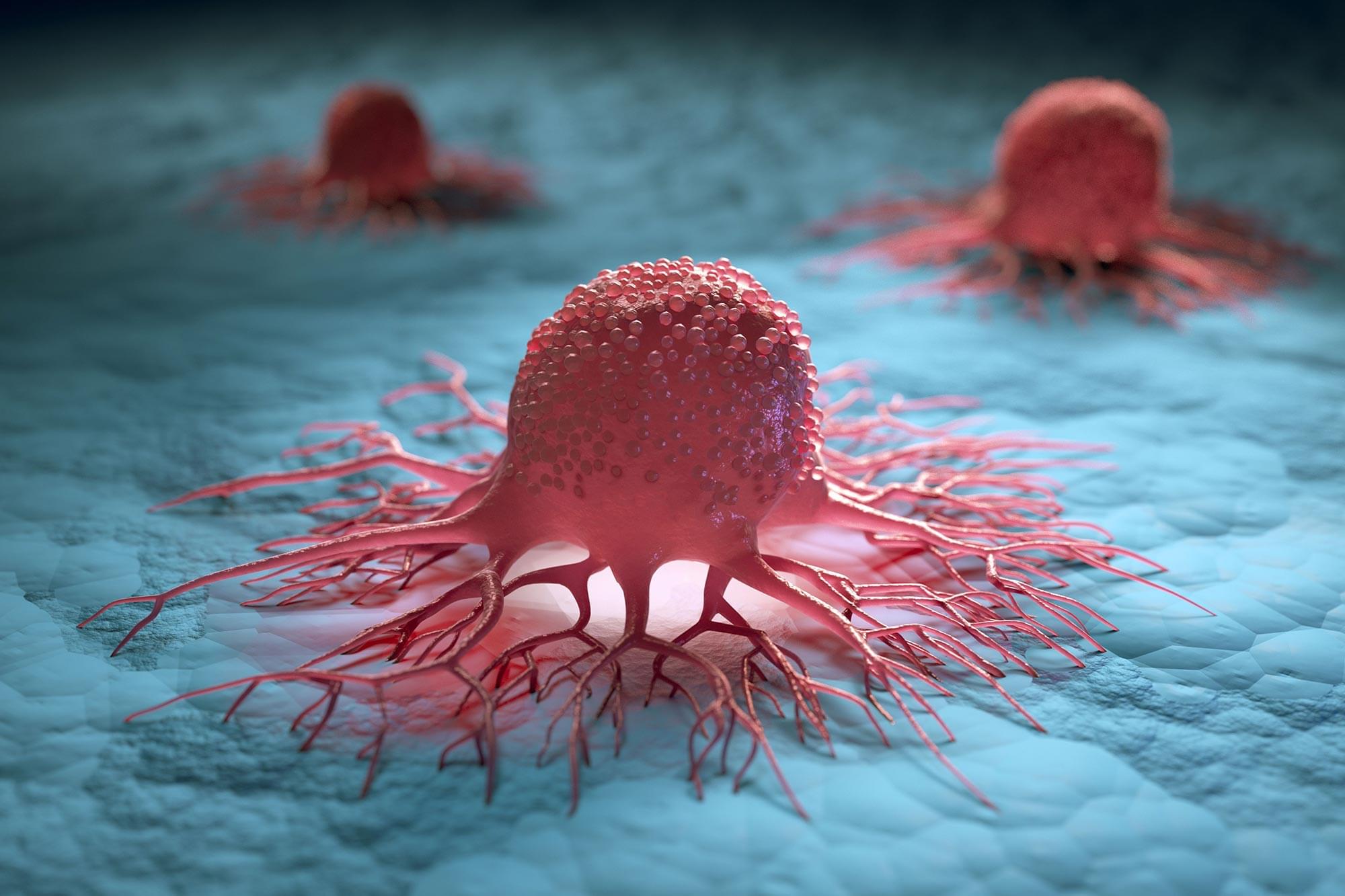
The discovery of a defensive mechanism could help stop cancer before it spreads. Cancer cells rapidly increase their energy output when physically squeezed, according to a study in Nature Communications. This immediate burst of energy is the first documented defensive response that helps cells repair DNA damage and endure the crowded conditions inside the human body.