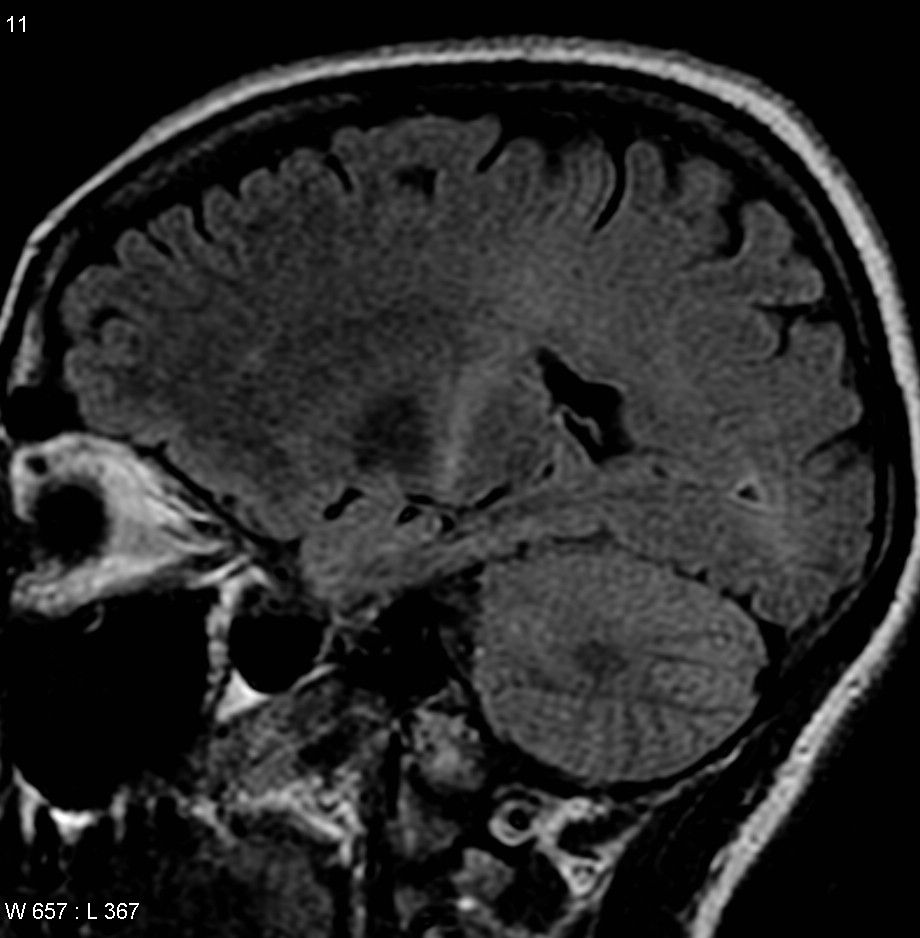
This study builds on an earlier paper by the Rothstein lab that looked at the most common genetic cause of ALS, a mutation in the C9orf72 gene (also referred to as the “C9 mutation”). There, they showed that the C9 mutation produced defects in a structure called the nuclear pore that is responsible for moving proteins and other molecules in and out of the nucleus of cells.
Amyotrophic lateral sclerosis (ALS) is a rapidly progressive and fatal degenerative disease affecting the nerve cells in the brain and spinal cord responsible for controlling voluntary muscle movement. “Sporadic” or non-inherited ALS, accounts for roughly 90% percent of cases, and 10% of cases are due to known genetic mutations. By studying lab-grown neurons derived from skin or blood cells from 10 normal controls, eight with an ALS causing mutation, and 17 with non-inherited ALS, researchers have found a possible starting point for the dysfunction that causes the disease. The study, which was published in Science Translational Medicine, was funded in part by the National Institute for Neurological Disorders and Stroke (NINDS), part of the National Institutes of Health.
Using a library of ALS patient-derived cells, the research team led by Jeffrey Rothstein, M.D., Ph.D., at Johns Hopkins University School of Medicine, Baltimore, developed induced pluripotent stem cell (iPSC)-derived neurons from the patients’ cultured cells to discover a common defect regardless of whether the cell came from persons with inherited or non-inherited ALS. They report that in ALS nerve cells, there is an accumulation of a protein called CHMP7 in the nucleus of cultured nerve cells as well as in ALS samples from the brain region that controls movement. Treatments that decrease the amount of CHMP7 in the cultured cells prevented a series of abnormalities that are characteristic of ALS.
“There is considerable interest in identifying new therapeutic targets for ALS, particularly for the sporadic form of the disorder,” said Amelie Gubitz, Ph.D., program director, NINDS. “Gene-targeting strategies like the one shown here now allow us to move from biological discovery straight to therapy development.”