AI is entering take-off mode just as we exit an economic downturn caused by the pandemic. Get ready for a productivity boom.

More TAME! The first part of this has a lot of result data.
Foresight Biotech & Health Extension Meeting sponsored by 100 Plus Capital.
2021 program & apply to join: https://foresight.org/biotech-health-extension-program/
Nir Barzilai, Albert Einstein School of Medicine.
TAME Q&A: Lessons for Progress on Aging.
About Nir Barzilai:
Nir Barzilai, MD, is a Professor in the Department of Endocrinology Medicine and the Department of Genetics at the Albert Einstein College of Medicine. He is also the Ingeborg and Ira Leon Rennert Chair of Aging Research at the Albert Einstein College of Medicine. Dr. Barzilai is the founding director of the Institute for Aging Research at Albert Einstein College of Medicine and the Director of the Nathan Shock Center for Excellence in the Basic Biology of Aging, funded by the National Institutes of Health (NIH); the center is coordinating 80 investigators and six program projects on the biology of aging. He is also the director of the Glenn Center of Excellence in the Biology of Human Aging. He is a chaired professor of medicine and of genetics and a member of the Diabetes Research Center and the divisions of endocrinology and geriatrics. Dr. Barzilai’s interests focus on several basic mechanisms in the biology of aging, including the biological effects of nutrients on extending life and the genetic determinants of life span. His team discovered many longevity gene in humans, and they further characterized the phenotype and genotype of humans with exceptional longevity through NIH awards. He also has an NIH Merit award investigating the metabolic decline that accompanies aging and its impact on longevity. Dr. Barzilai has published more than 270 peer-reviewed papers, reviews and chapters in textbooks. Dr. Barzilai serves on several editorial boards and advisory boards of pharmaceutical and start-up companies, and is a reviewer for numerous journals. A Beeson Fellow for Aging Research, Dr. Barzilai has received many other prestigious awards, including the Senior Ellison Foundation Award, the 2010 Irving S. Wright Award of Distinction in Aging Research, the NIA–Nathan Shock Award and a merit award from the NIA for his contributions in elucidating metabolic and genetic mechanisms of aging and was the 2018 recipient of the IPSEN Longevity award. He is leading the TAME (Targeting/Taming Aging with Metformin) Trial, a multi-center study to prove the concept that multi morbidities of aging can be delayed in humans and change the FDA indications to allow for next generation interventions. He is a founder of CohBar Inc. (now public company) and Medical Advisor for Life Biosciences. He is on the board of AFAR and a founding member of the Academy for Lifespan and Healthspan. He has been featured in major papers, TV programs, and documentaries (TEDx and TEDMED) and has been consulting or presented the promise for targeting aging at The Singapore Prime Minister Office, several International Banks, The Vatican, Pepsico, Milkin Institute, The Economist and Wired Magazine. His book, Age Later: Health Span, Life Span, and the New Science of Longevity, was published by St. Martin’s Press in June of 2020.
Zoom Transcription: https://otter.ai/u/0bz5o2crLQncfxlUkctY6NVzcCg.
“It’s an extraordinary paper with some extraordinary claims,” says Gray Camp, a developmental biologist at the University of Basel in Switzerland, whose lab last year reported2 growing brain organoids that contained a gene common to Neanderthals and humans. The latest work takes the research further by looking at gene variants that humans lost in evolution. But Camp remains sceptical about the implications of the results, and says the work opens more questions that will require investigation.
Humans are more closely related to Neanderthals and Denisovans than to any living primate, and some 40% of the Neanderthal genome can still be found spread throughout living humans. But researchers have limited means to study these ancient species’ brains — soft tissue is not well preserved, and most studies rely on inspecting the size and shape of fossilized skulls. Knowing how the species’ genes differ from humans’ is important because it helps researchers to understand what makes humans unique — especially in our brains.
The researchers, led by Alysson Muotri, a neuroscientist at the University of California, San Diego, used the genome-editing technique CRISPR–Cas9 to introduce the Neanderthal and Denisovan form of a gene called NOVA1 into human pluripotent stem cells, which can develop into any cell type. They cultured these to form organoids, clumps of brain-like tissue, up to 5 millimetres across, alongside normal human brain organoids for comparison.
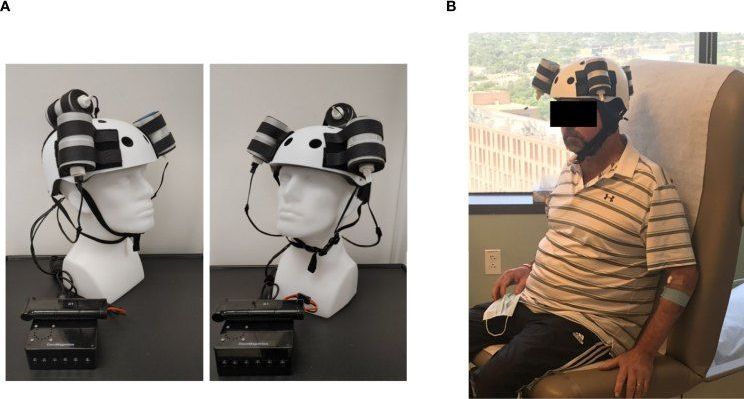
We’ve seen helmets and AI that can spot brain tumors, but a new hard hat can actually treat them, too.
As part of the latest neurological breakthrough, researchers used a helmet that generates a magnetic field to shrink a deadly tumor by a third. The 53-year-old patient who underwent the treatment ultimately died due to an unrelated injury, but an autopsy of his brain showed that the procedure had removed 31% of the tumor mass in a short time. The test marked the first noninvasive therapy for a deadly form of brain cancer known as glioblastoma.
The helmet features three rotating magnets connected to a microprocessor-based electronic controller operated by a rechargeable battery. As part of the therapy, the patient wore the device for five weeks at a clinic and then at home with the help of his wife. The resulting magnetic field therapy created by the helmet was administered for two hours initially and then ramped up to a maximum of six hours per day. During the period, the patient’s tumor mass and volume shrunk by nearly a third, with shrinkage appearing to correlate with the treatment dose.
4:47 BioAge, 8:10 Church talking about how controlling aging is no longer speculative, 10:44 urging caution as they are not really talking about turning 67 year olds into 20 year olds. Near the end Church mentions A.I. an exponential possibilities of hitting all the pathways at once.
Recently, Avi Roy, alongside Nathan Cheng & Laura Minquini, hosted the second Longevity Panel discussion, which assembled some of the brightest minds working on reversing aging, and enhancing health and life span.
As with the first event, this discussion was intended to illuminate how they are approaching longevity and to know if we are any closer in achieving it.
The talk was split into two sections: the first being open discussion guided by questions from the hosts. The talk was then opened up to the floor, allowing audience questions. Part 1 will provide the transcript from the first section of the Longevity Panel. Enjoy!
You can check out the full transcript, with addition links on the Gowing Life website:

The result is optogenetics, a mind-controlling technique that’s become one of neuroscience’s most popular tools. Here, scientists use genetic engineering to put different types of algae proteins into the brains of mice. They can then activate a neuron with an implanted fiber optic cable by pulsing certain wavelengths of light. These enhanced brain cells react as they would naturally, generating an electrical signal that’s passed down and interpreted by the mouse’s brain.
Sound familiar?
If an algae protein can artificially allow neurons in the brain to translate light into electrical information, why can’t it do the same for damaged eyes?

I know that this is controversial in the longevity community, but there was overwhelming agreement among the dentists I talked with that mouthwash is excellent for preventing gingivitis (one study found that it was more effective than flossing) and reducing plaque.
Are you working to extend your healthspan and lifespan? Address the most common aging teeth problems with these dentist-approved tricks.
Improving Quality Of Life & Health, For Hundreds Of Millions Globally, Suffering Food Allergies & Intolerances — Lisa Gable, Chief Executive Officer, Food Allergy Research & Education (FARE)
Lisa Gable is the Chief Executive Officer, of Food Allergy Research & Education (FARE — https://www.foodallergy.org), an organization with a mission to improve the quality of life and the health of 85 million Americans with food allergies and food intolerances, including 32 million of those are at risk for life-threatening anaphylaxis, and to provide them hope through the promise of new treatments. To date FARE has turned over $100 million in donor gifts into ground-breaking research and has provided a voice for the community, advocating on its behalf and offering hope for a better tomorrow.
Ms. Gable has served four U.S. presidents and two governors, counseled Fortune 500 CEOs, and represented global public-private partnerships and non-profits with an end goal of moving organizations to higher levels of performance.
As the former President of the Healthy Weight Commitment Foundation, Ms. Gable created and led a coalition of food and beverage industry corporations and public health and government agencies, resulting in the reduction of 6.4 trillion calories from the American diet.
Ms Gable was appointed the first female U.S. Commissioner General to the 2005 Aichi World EXPO, holding the personal rank of Ambassador, served as a U.S. Delegate to the United Nations Commission on the Status of Women, and served both in the Reagan White House and Defense Department, serving as an advisor to the Secretary of Defense and the Joint Chief of Staff.

I believe since the weird localization of the virus in Japan that this sorta self evolving virus needs to have a counter like a self evolving vaccine but at the very least new variants can be made super fast with new technology in vaccine making.
Washington — Dr. Scott Gottlieb, former Food and Drug Administration commissioner, said Sunday he thinks the U.S. is further into the COVID-19 epidemic driven by the Delta variant than Centers for Disease Control and Prevention (CDC) models are picking up. He said that could mean “hopefully we’re going to turn a corner” in the next two to three weeks.
“We’re not doing a lot of testing. More of the testing that we are doing is antigen tests that are being done at home and not getting reported,” Gottlieb said. “So, I think we’re much further into this epidemic than we’re picking up and hopefully further through this epidemic.”