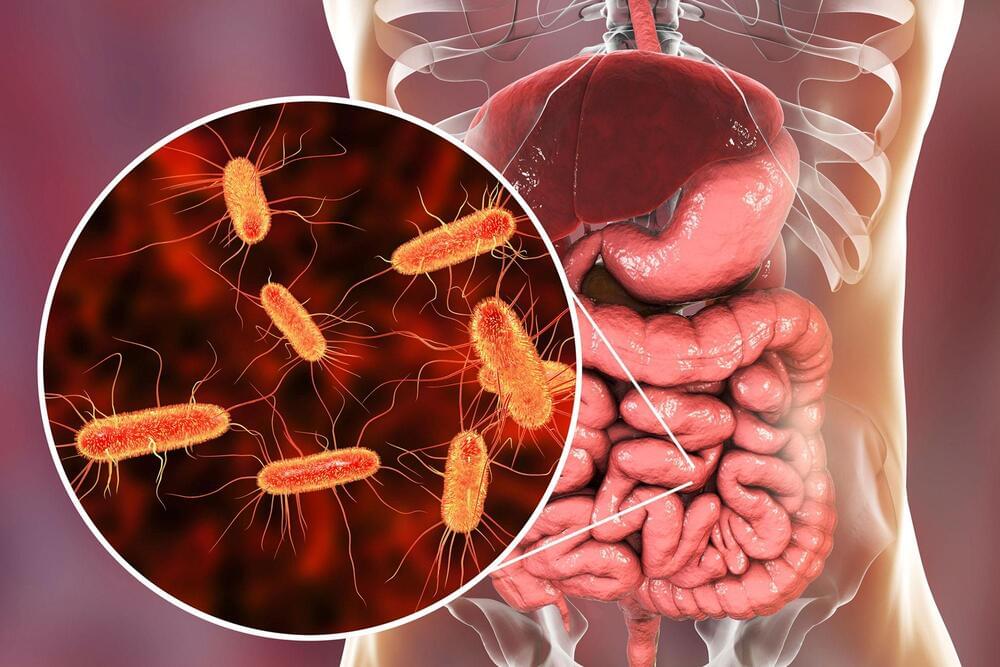
Researchers at the University of Colorado School of Medicine have found that a unique bacteria found in the gut may be responsible for causing rheumatoid arthritis (RA) in patients who are already predisposed to the autoimmune disease.
A group of researchers from the Division of Rheumatology worked on the study under the leadership of Kristine Kuhn, MD, Ph.D., an associate professor of rheumatology. The study was recently published in the journal Science Translational Medicine. Meagan Chriswell, a medical student at CU, is the paper’s lead author.
“Work led by co-authors Drs. Kevin Deane, Kristen Demoruelle, and Mike Holers here at CU helped establish that we can identify people who are at risk for RA based on serologic markers, and that these markers can be present in the blood for many years before diagnosis,” Kuhn says. “When they looked at those antibodies, one is the normal class of antibody we normally see in circulation, but the other is an antibody that we usually associate with our mucosa, whether it be the oral mucosa, the gut mucosa, or the lung mucosa. We started to wonder, ‘Could there be something at a mucosal barrier site that could be driving RA?’”