Metamorworks/iStock.
Safe and helpful answers.

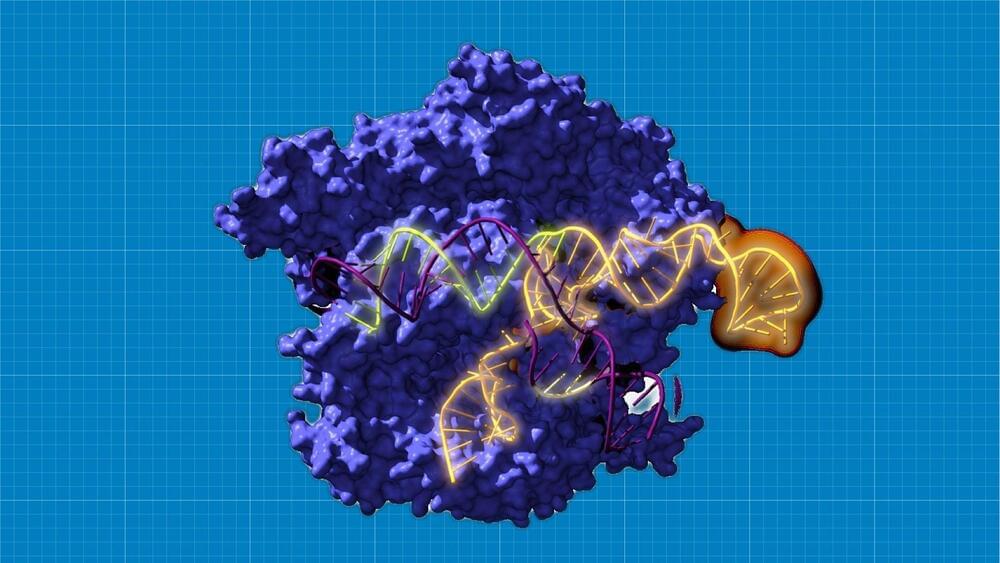
“This research signifies an extraordinary advance in knowledge about the origin and evolution of CRISPR-Cas systems.”
An international research team reconstructed the CRISPR-Cas system for the first time, dating back to 26 billion years ago. Their findings imply that the revived systems are functional and more adaptable than the previous iterations.
Led by teams from the Spanish National Research Council, the University of Alicante, the Rare Diseases Networking Biomedical Research Center (CIBERER), the Ramón y Cajal Hospital-IRYCIS, and other national and international institutions are working with Ikerbasque research professor Rául Pérez-Jiménez of CIC nanoGUNE.
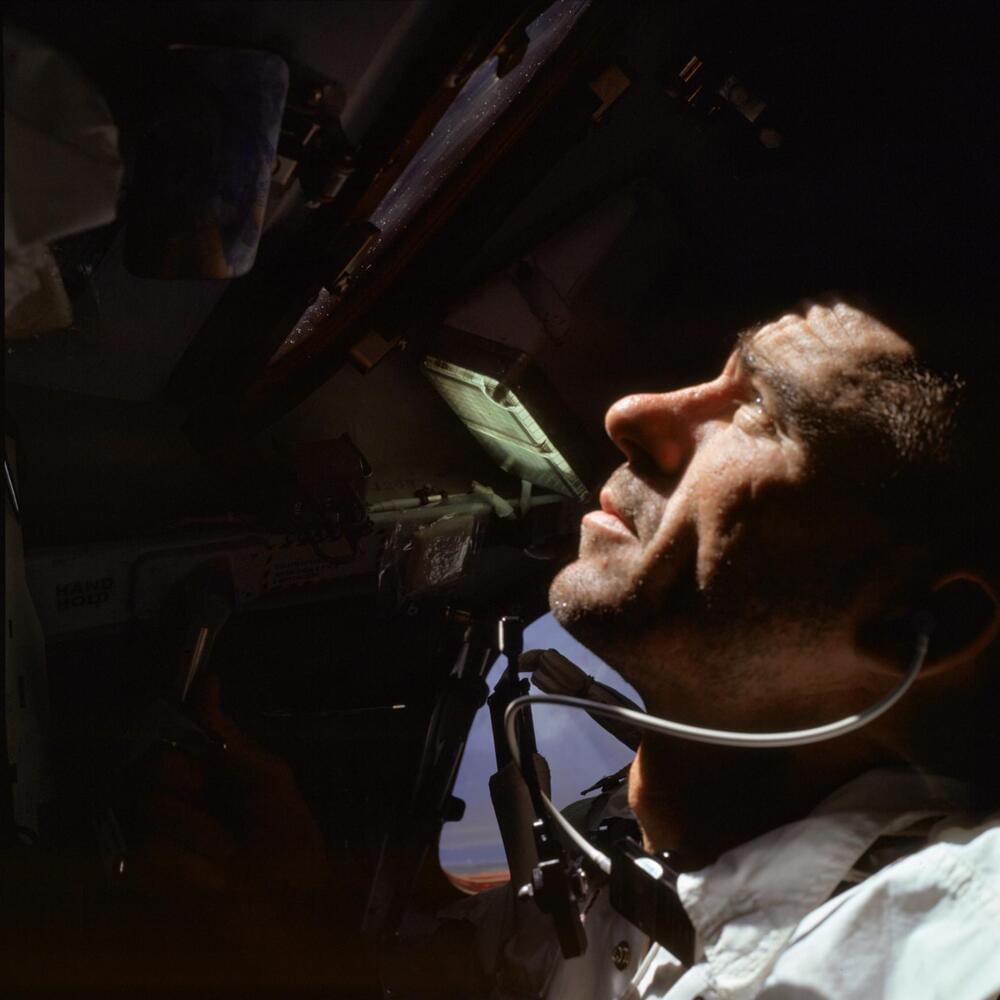
He was also a physicist and a night fighter pilot.
Walter Cunningham, NASA astronaut who flew on Apollo 7, an 11-day mission manned mission in 1968 that paved the way for the first human landing on the Moon, has died at the age of 90, NASA said on its website. Cunningham died of natural causes at a hospital in Houston on Tuesday.
Born in 1932, Walter Cunningham joined the U.S. Navy in 1951, where he served in the U.S. Marine Corps and flew 54 missions as a night fighter pilot In Korea before retiring at the rank of a colonel. He then graduated with Honors in Physics in 1960 and followed it up with a distinction in Physics a year later at the University of California at Los Angeles. astronaut Walter Cunningham, who flew into space on Apollo 7, the first flight with crew in NASA’s Apollo Program, died early Tuesday morning in Houston. He was 90 years old.

In 2021, one of the record years in biotechnology investments, about 50 drugs were approved by the FDA. 36 of these were small molecules. In my opinion, only about 5 of these were truly innovative targeting novel mechanisms and novel targets. The 10th revision of the International Classification of Diseases (ICD) contained 55,000 codes for diseases, injuries and conditions. Even if we assume that there are only 10,000 diseases, 50 FDA approvals per year seems to be an extraordinarily small number.
The reason for this small number of innovative and effective therapeutics is the long time, high cost, and low probability of success of drug discovery and development. On average, this process takes 12 years, costs over $2 Billion dollars and fails over 90% of the time. The most innovative therapeutics with novel targets have even higher probability of failure. To get these 50 drugs approved in 2021, the pharmaceutical companies globally spent over $100 Billion and over a decade.
The biotechnology industry is very different from any other industry and it is important to understand how it works, and the role China plays in delivering safe and effective medicines to suffering patients worldwide. Sharing risk, expenses, and infrastructure will result in acceleration of global biotechnology and increase the number of innovative drug approvals. Closer collaboration between the US and China in biotechnology would allow investors to share huge risks and returns while benefiting everyone on the planet and making this world a much better place.

Despite surges in fields like AI, medicine and nuclear energy, major advances in science and technology are slowing and are fewer and farther between than decades ago, according to a study published in Nature.
The researchers analyzed some 45 million scientific papers and 3.9 million patents between 1945 and 2010, examining networks of citations to assess whether breakthroughs reinforced the status quo or disrupted existing knowledge and more dramatically pushed science and technology off into new directions.
Across all major scientific and technological fields, these big disruptions—the discovery of the double helix structure of DNA, which rendered earlier research obsolete, is a good example of such research—have become less common since 1945, the researchers found.
Ray Kurzweil, an American Jewish inventor and futurist, claims that within ten years, man will be able to defeat old age and death thanks to the accelerated development of technology.
My question in relation to Kurzweil’s statement is: What is so good about us constantly living all the time? Why live at all if we are never to die?
On the contrary, if we attain the purpose of our lives while we are alive, then we will reach a spiritual, eternal, and perfect state, i.e. one where we will have no feeling of a lack. In our current lives, we constantly live out of feeling lack and the need to fulfill our lacks. However, we can reach a state where we have no such feeling of a lack, but that we have an abundance of everything.
Developing ourselves spiritually has nothing to do with medicine or technology. It has to do with our inner world, i.e. with how we feel that we can give and receive from everyone, and live in a world that is boundless, with no beginning or end. Then, even if our bodies die, we will not feel it as death.
Follow Kabbalist Dr. Michael Laitman:
Twitter ► https://twitter.com/laitman.
Facebook ► https://facebook.com/michaellaitman.
Instagram ► https://www.instagram.com/dr_michaellaitman.
YouTube ► https://youtube.com/michaellaitman.
Quora ► https://quora.com/profile/Kabbalist-Dr-Michael-Laitman.
Medium ► https://medium.com/@michaellaitman.
Thrive Global ► https://thriveglobal.com/authors/michael-laitman/
LinkedIn ► https://il.linkedin.com/in/michaellaitman.
NewsMax ► https://www.newsmax.com/insiders/michaellaitman/id-504
The Times of Israel ► https://blogs.timesofisrael.com/author/michael-laitman.
Jerusalem Post ► https://jpost.com/Author/Michael-Laitman.
Ha’aretz ► https://haaretz.com/haaretz-labels/laitman.
Breaking Israel News ► https://breakingisraelnews.com/author/michael_laitman.
Laitman Kabbalah Publishers (Books) ► https://kabbalahbooks.info.
Official Website ► https://michaellaitman.com.
This post was written by the editor of Kabbalist Dr. Michael Laitman’s YouTube channel.
In this episode, David and Peter discuss aging as a disease, the technology needed to reverse aging, and tips and tricks to increase your lifespan.
David Sinclair is a biologist and academic known for his expertise in aging and epigenetics. Sinclair is a genetics professor and the Co-Director of Harvard Medical School’s Paul F. Glenn Center for Biology of Aging Research. He’s been included in Time100 as one of the 100 Most Influential People in the World, and his research has been featured all over the media. Besides writing a New York Times Best Seller, David has co-founded several biotech companies, a science publication called Aging, and is an inventor of 35 patents.
Read David’s book, Lifespan: Why We Age-and Why We Don’t Have To: https://a.co/d/85H3Mll.
This episode is brought to you by Levels: real-time feedback on how diet impacts your health. https://levels.link/peter.
Consider a journey to optimize your mind and body by visiting http://mylifeforce.com/peter.
Read the Tech Blog: https://www.diamandis.com/blog.
Timestamps.