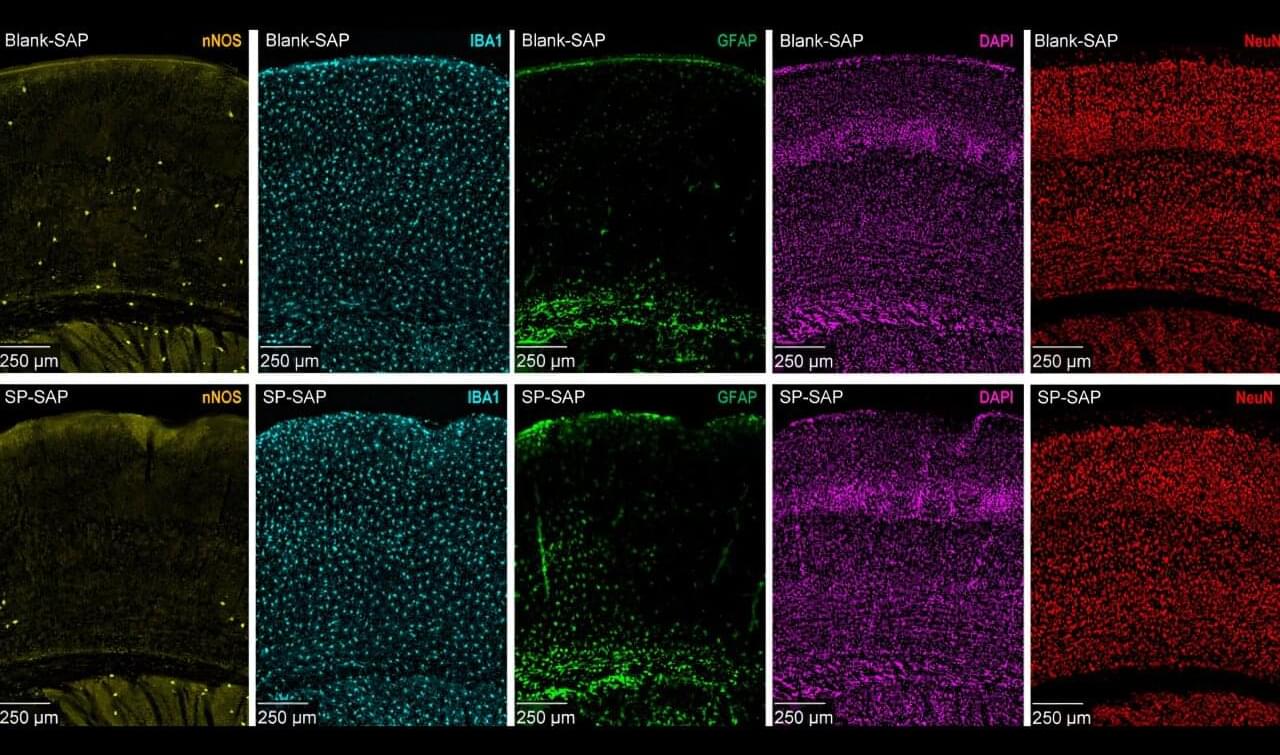
It sounds like a strange online myth, but scientists in Japan have been studying whether mammals, including humans, can absorb oxygen through the gut. This phenomenon, often called butt breathing, is officially known as enteral ventilation. In a world where lung failure and ventilator shortages can quickly turn deadly, this idea could change how emergency oxygen therapy works. According to a peer-reviewed study published in Med (Cell Press), researchers at Tokyo Medical and Dental University successfully demonstrated gut-based oxygen absorption in mice and pigs, sparking global curiosity. You can read the full study. The experiment may sound unconventional, but it could one day save lives when traditional breathing support is unavailable…
…Respiratory failure remains one of the most difficult emergencies to manage in modern medicine. Mechanical ventilators save countless lives, but they can also cause lung damage and are not always accessible in low-resource settings. Enteral ventilation could provide an alternative when ventilators are unavailable or when lungs are too damaged to function effectively.
…Despite its promise, the approach still faces several obstacles before it can reach clinical use. The recent human study only confirmed safety, not effectiveness. Researchers now need to show that oxygen introduced through the colon can significantly raise blood oxygen levels.
Scientists in Japan are exploring a novel way to deliver oxygen through the gut. This method, called enteral ventilation, involves introducing oxygen-rich liquid rectally. Early animal trials show promise, and a human safety study found it well-tolerated. This could offer a vital backup for patients with severe breathing difficulties when ventilators are unavailable.