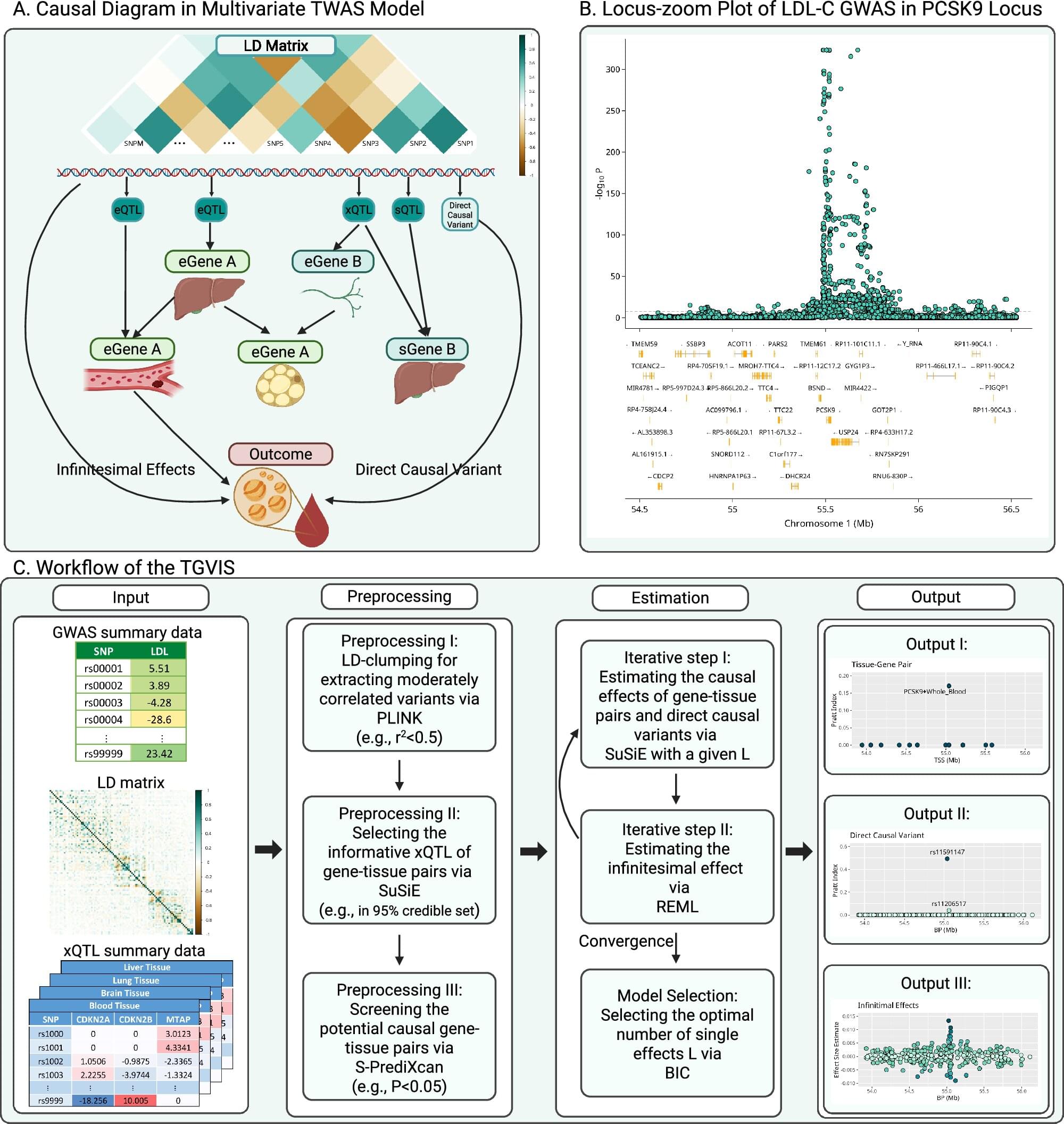
Genetic changes can signal evidence of disease, but pinpointing which genes and what’s changed can be difficult.
But in a study of traits that offer clues to a person’s cardiovascular health —such as lipid and glucose levels and inflammation—a team of researchers at Case Western Reserve University devised a computational method and tool to improve how genes and genetic changes that cause diseases are identified.
Their new approach could allow doctors to detect and treat so-called cardiometabolic diseases earlier in their development. Their findings were recently published in the journal Nature Communications.