Revolutionizing Cancer Research: The Power of Nanobiotechnology|Role of nanotechnology in Cancer.
#cancer #biotechnology #nanotechnology #nanobiotechnology #cancerbiology #cancerresearch #biology #molecularbiology #molelixirinformatics.
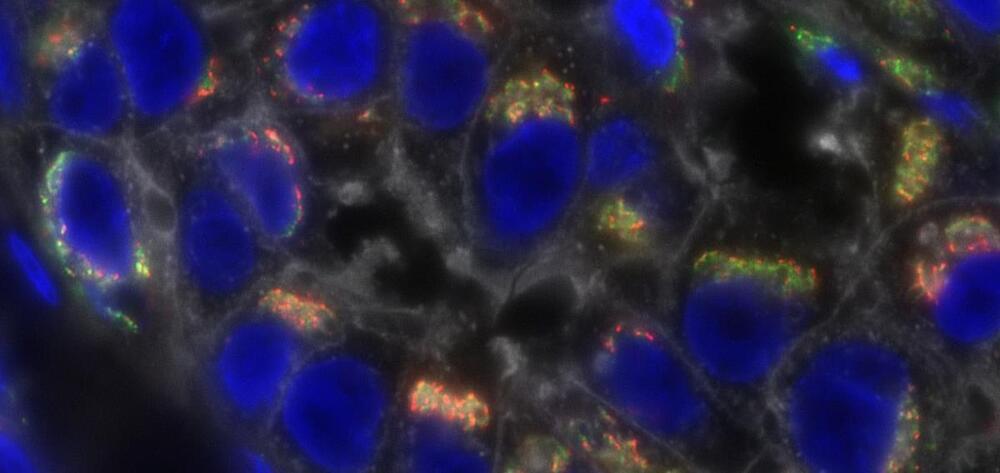
✓Discover the exciting advancements in Nanobiotechnology and its role in transforming cancer research.
✓This groundbreaking field is using tiny nanotechnology to make a big impact in the fight against cancer. From early detection to targeted treatments, Nanobiotechnology is providing new hope for a future free from the disease.
✓ Join us in this informative and educational video as we take you on a journey to understand the power of nanotechnology in cancer research.
Learn about the latest innovations, the potential benefits, and the impact it could have on our lives.
✓Get ready to be inspired and informed about this amazing field.
For more details: