In a promising advance in its fight against cervical cancer, India recently launched its first locally produced version of the human papillomavirus (HPV) vaccine “Cervavac”. Currently, India lacks a national immunization program for carcinoma cervix eradication. Inclusion of Cervavac into the national immunization schedule will undoubtedly boost the fight against cervical cancer.
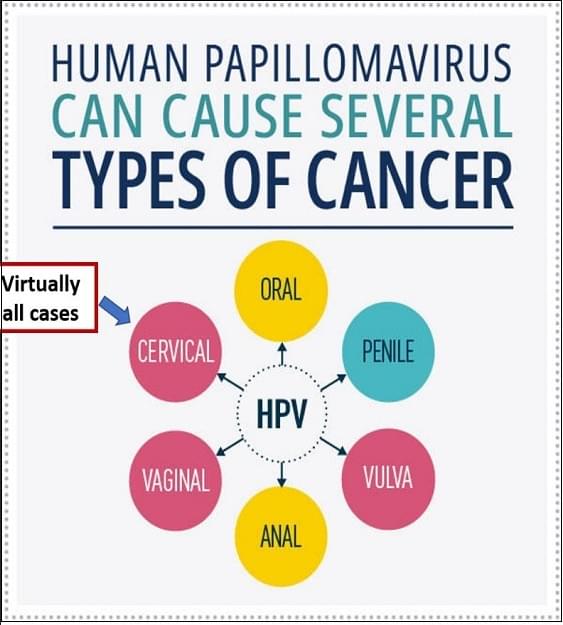
HPV is a group of more than 200 related viruses, sexually transmitted HPV types fall into two groups, low risk and high risk. High-risk HPVs can cause several types of cancer. HPV infection is common. Nearly all sexually active people are infected with HPV within months to a few years of becoming sexually active. Most HPV infections don’t cause cancer. Our immune system usually clears most of HPV infections. Only about 1% of High-risk HPV infections that persist can cause cancer. Human papillomavirus (HPV) infection is a well-established cause of cervical cancer and there is growing evidence of HPV being an important factor in other anogenital cancers (anus, vulva, vagina, and penis) as well as head and neck cancers.