As the coming genetic revolution plays out, we’ll still have sex for most of the same reasons we do today. But we’ll increasingly not do it to procreate.
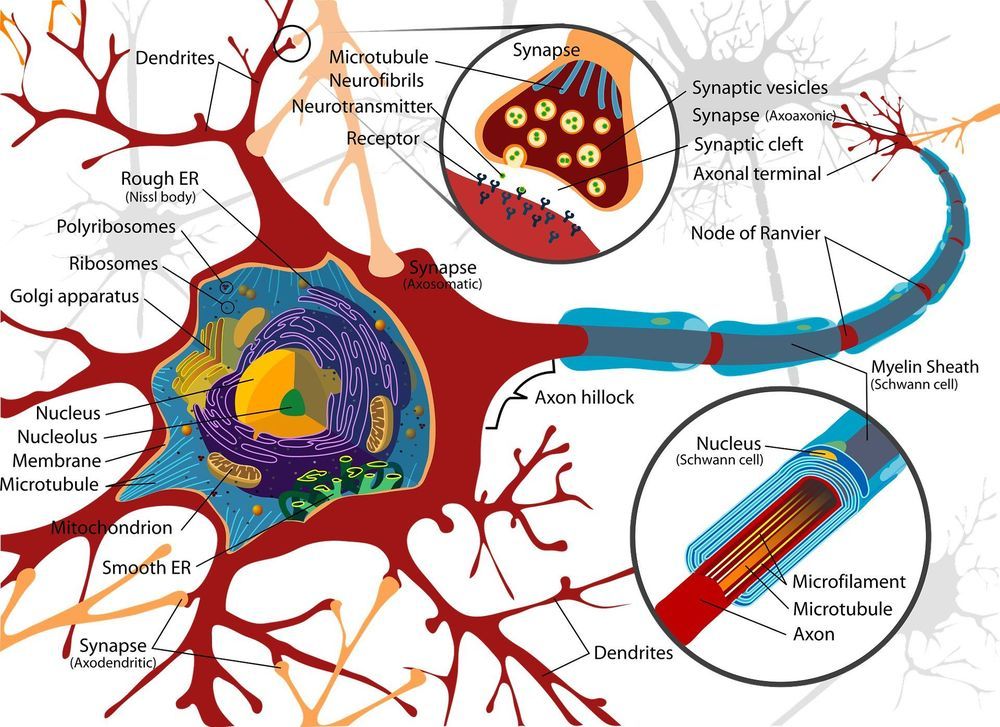
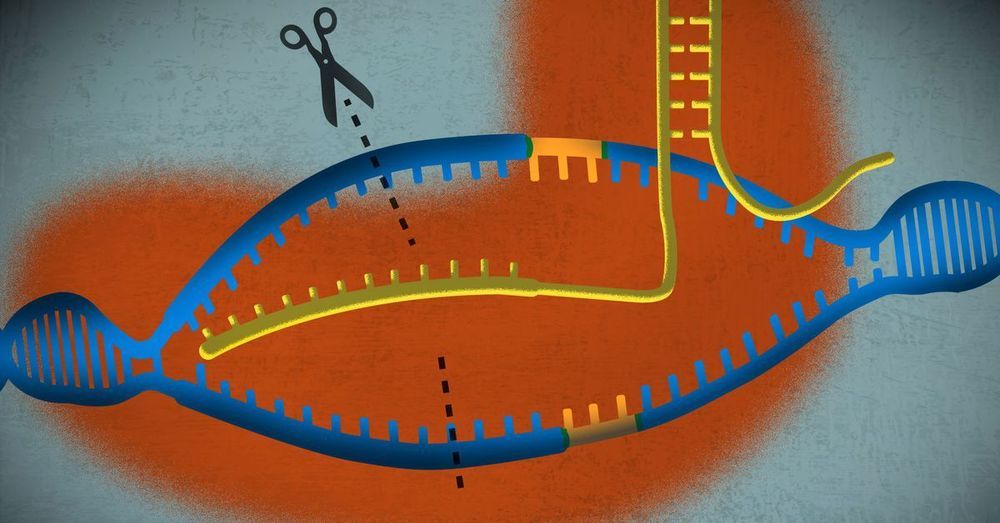
Another rocket booster will be the application of gene editing technologies like CRISPR to edit the genomes of pre-implanted embryos or of the sperm and eggs used to create them. Just this week, Chinese researchers announced they had used CRISPR to edit the CCR5 gene in the pre-implanted embryos of a pair of Chinese twins to make them immune to HIV, the first ever case of gene editing humans and a harbinger of our genetically engineered future. The astounding complexity of the human genome will put limits on our ability to safely make too many simultaneous genetic changes to human embryos, but our ability and willingness to make these types of alterations to our future children will grow over time along with our knowledge and technological ability.
With so much at stake, prospective parents will increasingly have a stark choice when determining how to conceive their children. If they go the traditional route of sex, they will experience both the benign wisdom and unfathomable cruelty of nature. If they use IVF and increasingly informed embryo selection, they will eliminate most single gene mutation diseases and likely increase their children’s chances of living a longer and healthier life with more opportunity than their unenhanced peers. But the optimizing parents could also set up their children for misery if these children don’t particularly enjoy what they have been optimized to become or see themselves as some type of freakish consumer product with emotions.
But although there will be pros and cons on each side, the fight between conception through good old-fashioned sex and conception in the lab will ultimately not be fair. Differences and competition within and between societies will pressure parents and societies to adopt ever more aggressive forms of reproductive technology if they believe doing so will open possibilities and create opportunities for the next generations rather than close them.