Tesla will want you to pay $5,000 due immediately upon reserving, then another $15,000 within 10 days to secure your order.



Summary: Mouse study reveals exercise increases dopamine signaling in motor areas of the brain. The findings may explain why exercise eases symptoms of Parkinson’s disease.
Source: SfN
Exercise increases dopamine signaling in the motor areas of mice, according to research recently published in Journal of Neuroscience.

(Texas Tribune/KXAN) — A massive security breach at the Texas Department of Insurance leaked the personal information of almost 2 million Texans for nearly three years, according to a state audit released last week.
The department said the personal information of 1.8 million workers who have filed compensation claims — including Social Security numbers, addresses, dates of birth, phone numbers and information about workers’ injuries — was accessible online to members of the public from March 2019 to January 2022.
Though personal information was compromised – the agency now says there’s no reason to believe the data was used.
Never mind reading generic guides or practicing with friends — Google is betting that algorithms can get you ready for a job interview. The company has launched an Interview Warmup tool that uses AI to help you prepare for interviews across various roles. The site asks typical questions (such as the classic “tell me a bit about yourself”) and analyzes your voiced or typed responses for areas of improvement. You’ll know when you overuse certain words, for instance, or if you need to spend more time talking about a given subject.
Interview Warmup is aimed at Google Career Certificates users hoping to land work, and most of its role-specific questions reflect this. There are general interview questions, though, and Google plans to expand the tool to help more candidates. The feature is currently only available in the US.
AI has increasingly been used in recruitment. To date, though, it has mainly served companies during their selection process, not the potential new hires. This isn’t going to level the playing field, but it might help you brush up on your interview skills.

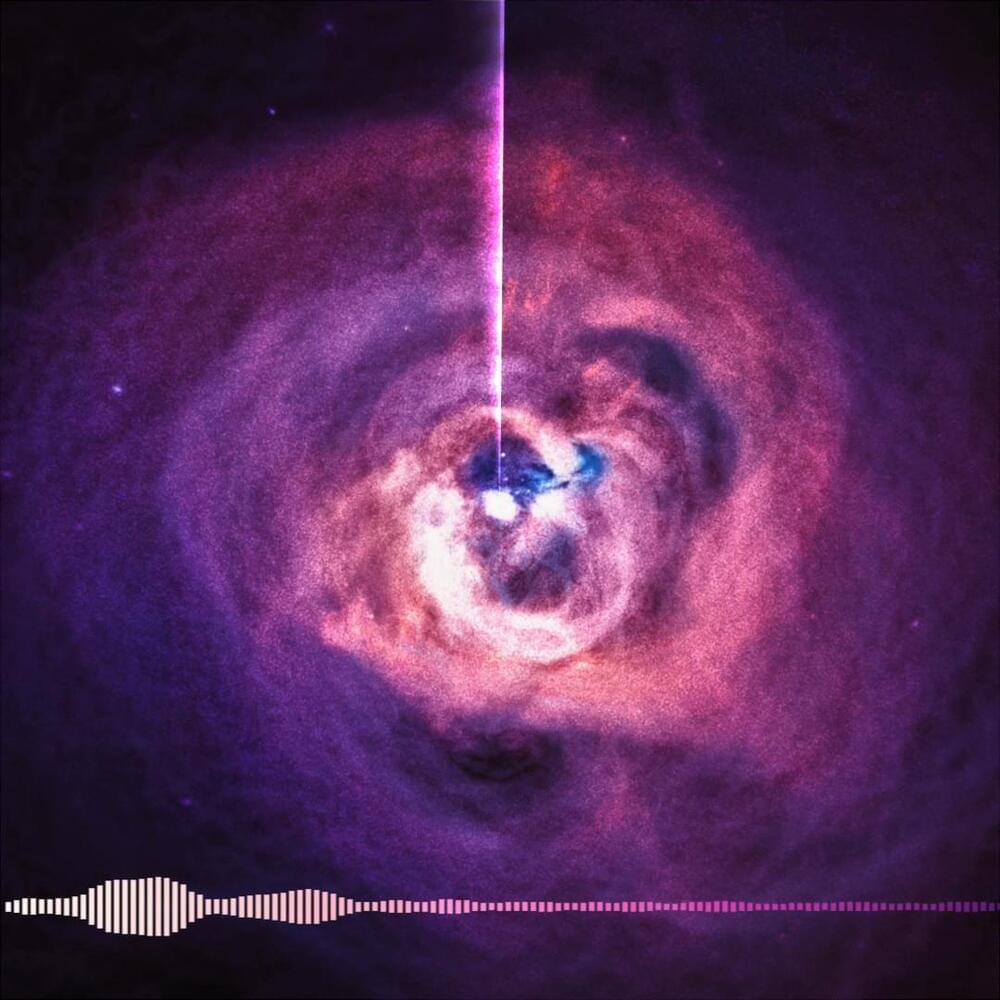
Hello darkness, my old friend 🎵
Want to listen to a black hole? The black hole at the center of the Perseus galaxy cluster sends out pressure waves that ripple the cluster’s gas, which we can translate into a note. #BlackHoleWeek https://go.nasa.gov/3MQae1I



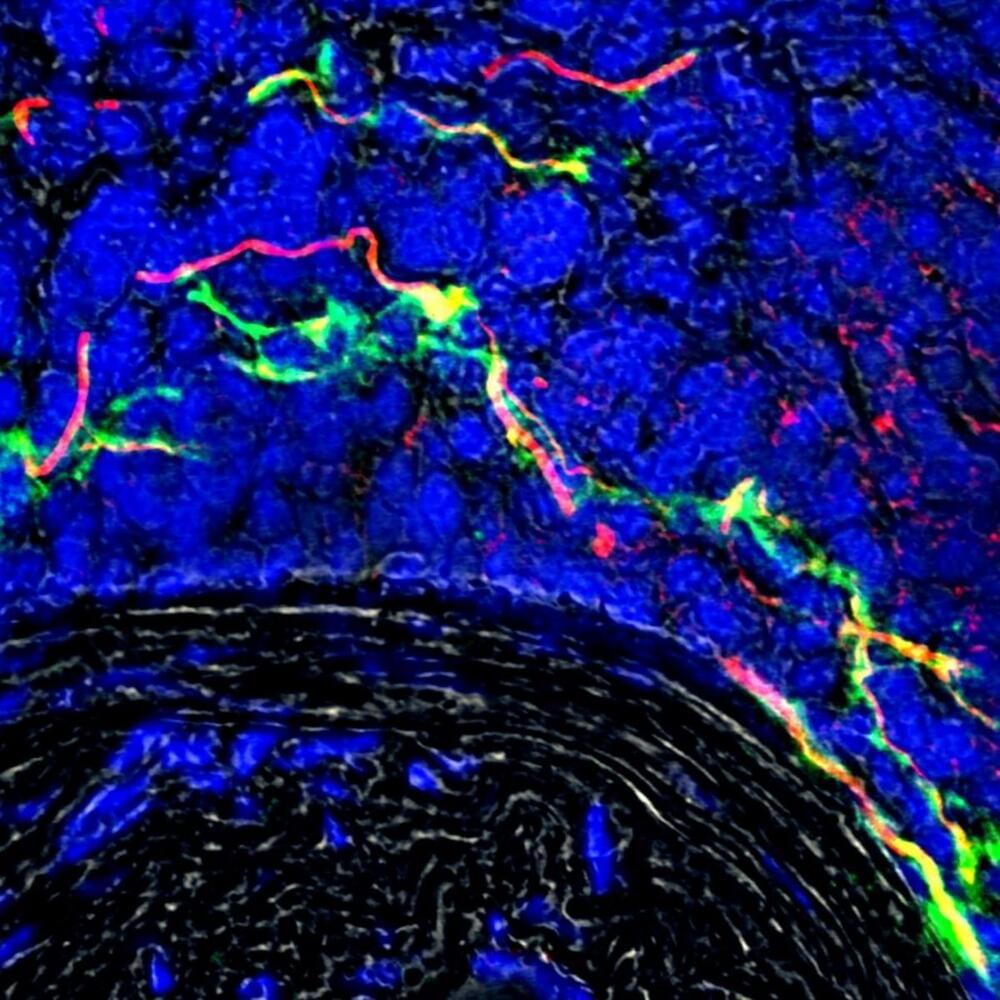
An international team which includes University of Manchester scientists has for the first time demonstrated that nerve signals are exchanged between clogged up arteries and the brain.
The discovery of the previously unknown electrical circuit is a breakthrough in our understanding of atherosclerosis, a potentially deadly disease where plaques form on the innermost layer of arteries.
The study of mice found that new nerve bundles are formed on the outer layer of where the artery is diseased, so the brain can detect where the damage is and communicate with it.