Regenerative responses in salivary gland organoids after irradiation are mediated by IFN-I signaling.

Developed at Caltech, a new robot is a humanoid that can launch an M4 drone, switching between different modes of motion, with wheels that can become rotors.

In 1887, one of the most important experiments in the history of physics took place. American scientists Michelson and Morley failed to measure the speed of Earth by comparing the speed of light in the direction of Earth’s motion with that perpendicular to it. That arguably most important zero measurement in the history of science led Einstein to postulate that the speed of light is constant and consequently to formulate his theory of special relativity.
This theory implies that all laws of physics are the same, independent of the relative motion between observers—a concept known as Lorentz invariance.
Meanwhile, quantum theory has been developed, with Lorentz invariance at the heart of all its theoretical frameworks, in particular quantum field theory and the Standard Model of Particle Physics. The latter is the most precisely tested theory ever developed and has been verified to incredible precision.

A Princeton team built a new tantalum-silicon qubit that survives for over a millisecond, far surpassing today’s best devices. The design tackles surface defects and substrate losses that have limited transmon qubits for years. Easy to integrate into existing quantum chips, the approach could make processors like Google’s vastly more powerful.

“You should have a few good years ahead of you but I wouldn’t hold my Bitcoin,” Peronnin said, laughing. “They need to fork [move to a stronger blockchain] by 2030, basically. Quantum computers will be ready to be a threat a bit later than that,” he said.
Quantum doesn’t just threaten Bitcoin, of course, but all banking encryption. And it is likely that in all these cases companies are developing quantum resistant tools to upgrade their existing security systems.
Defensive security algorithms are improving, Peronnin said, so it’s not certain when the blockchain will become vulnerable to a quantum attack. But “the threshold for such an event is coming closer to us year by year,” he said.

“Drains” in the brain, responsible for clearing toxic waste in the organ, tend to get clogged up in people who show signs of developing Alzheimer’s disease, a study by researchers from Nanyang Technological University, Singapore (NTU Singapore) has discovered.
This suggests that such clogged drains, a condition known as “enlarged perivascular spaces,” are a likely early-warning sign for Alzheimer’s, a common form of dementia.
“Since these brain anomalies can be visually identified on routine magnetic resonance imaging (MRI) scans performed to evaluate cognitive decline, identifying them could complement existing methods to detect Alzheimer’s earlier, without having to do and pay for additional tests,” said Associate Professor Nagaendran Kandiah from NTU’s Lee Kong Chian School of Medicine (LKCMedicine) who led the study.
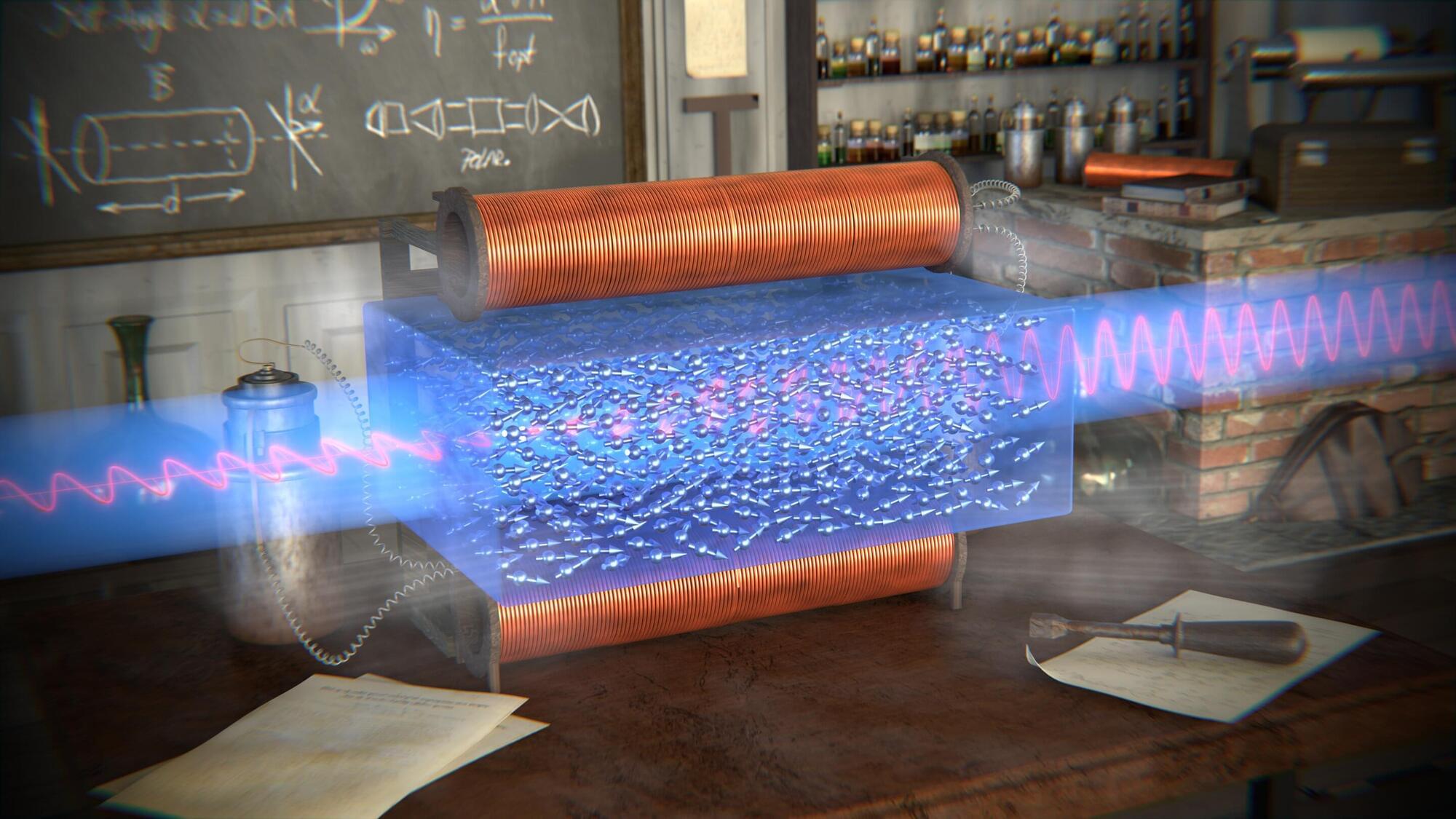
Researchers at the Hebrew University of Jerusalem discovered that the magnetic component of light plays a direct role in the Faraday effect, overturning a 180-year-old assumption that only its electric field mattered.
Their findings, published in Scientific Reports, show that light can magnetically influence matter, not just illuminate it. The discovery opens new possibilities in optics, spintronics, and quantum technologies.
The study was led by Dr. Amir Capua and Benjamin Assouline from the Institute of Electrical Engineering and Applied Physics at the Hebrew University of Jerusalem. It presents the first theoretical proof that the oscillating magnetic field of light directly contributes to the Faraday effect, a phenomenon in which the polarization of light rotates as it passes through a material exposed to a constant magnetic field.
321 watching now • Started streaming on Nov 17, 2025 • #news #latestnews #economictimes
This is a ~1 hour 20 min talk and discussion titled(https://hameroff.arizona.edu/).