China’s researchers stabilized a 100 MW microgrid, integrating a small modular reactor (SMR) and solar power.



Earlier in 2025, Chinese solar manufacturer Longi announced it had built the world’s most efficient solar cell. The hybrid interdigitated back-contact (HIBC) cell achieved 27.81% efficiency, which was verified by Germany’s Institute for Solar Energy Research Hamelin (ISFH).
Now, in a paper published in the journal Nature, researchers are sharing the technical details of their breakthrough.
For solar technology to deliver on its promise, solar cells and panels must convert as much sunlight as possible into energy. Typically, standard cells achieve up to 26% efficiency, that is, they convert 26% of the sunlight hitting them into electrical energy.
To capture more of the Sun’s spectrum, Steve Albrecht of the Technical University of Berlin and the Helmholtz Centre for Materials and Energy added a third layer of perovskite to make a so-called triple-junction cell, which could potentially offer even higher efficiencies. “It is truly a product of the future,” he says.
Other researchers are teaming perovskites with organic solar cells, forming flexible tandems suitable for indoor applications, or to cover vehicles. Yi Hou of the National University of Singapore points out that the perovskite layer filters ultraviolet light that would damage the organic cell. His team made a flexible perovskite–organic tandem5 with a record efficiency of 26.7%, and he is commercializing the technology through his company Singfilm Solar.
Despite the promising efficiency results, there was broad consensus at the conference that long-term stability is the field’s most pressing issue. Collaboration between researchers from academia, industry and national labs will be vital to fix that, says Marina Leite at the University of California, Davis: “We can work together to finally resolve the problem of stability in perovskites and truly enable this technology in the near future.”

Nissan just announced a solar-powered EV based on the Nissan Sakura for this year’s Japan Mobility Show.
Built using the super popular kei car as a platform, the solar-powered Sakura promises ‘free’ motoring thanks to its solar panels.
In theory, you can drive it for a year without ever plugging it in.

Graphene is a remarkable “miracle” material, consisting of a single, atom-thin layer of tightly connected carbon atoms that remains both stable and highly conductive. These qualities make it valuable for many technologies, including flexible screens, sensitive detectors, high-performance batteries, and advanced solar cells.
A new study, carried out by the University of Göttingen in collaboration with teams in Braunschweig and Bremen in Germany, as well as Fribourg in Switzerland, shows that graphene may be even more versatile than previously believed.
For the first time, researchers have directly identified “Floquet effects” in graphene. This finding settles a long-running question: Floquet engineering – an approach that uses precise light pulses to adjust a material’s properties – can also be applied to metallic and semi-metallic quantum materials like graphene. The work appears in Nature Physics.

The sun produces more power than 100 trillion times humanity’s entire electricity generation. In orbit, solar panels can be eight times more productive than their Earth-bound counterparts, generating energy almost continuously without the need for heavy battery storage. These facts have led a team of Google researchers to ask what if the best place to scale artificial intelligence isn’t on Earth at all, but in space?
Project Suncatcher, Google’s latest space mission, envisions constellations of solar-powered satellites equipped with processors and connected by laser-based optical links. The concept tackles one of AI’s most pressing challenges, the enormous energy demands of large-scale machine learning systems, by tapping directly into the solar system’s ultimate power source. A new research paper published by Google describes their progress toward addressing the technical challenges.
The proposed system would operate in a sun-synchronous low Earth orbit, where satellites remain in almost constant sunlight. This orbital choice maximizes solar energy collection while minimizing battery requirements. However, making space-based AI infrastructure viable requires solving several formidable engineering challenges.
MIT researchers have developed a lightweight polymer film that is nearly impenetrable to gas molecules, raising the possibility that it could be used as a protective coating to prevent solar cells and other infrastructure from corrosion, and to slow the aging of packaged food and medicines.
The polymer, which can be applied as a film mere nanometers thick, completely repels nitrogen and other gases, as far as can be detected by laboratory equipment, the researchers found. That degree of impermeability has never been seen before in any polymer, and rivals the impermeability of molecularly-thin crystalline materials such as graphene.
“Our polymer is quite unusual. It’s obviously produced from a solution-phase polymerization reaction, but the product behaves like graphene, which is gas-impermeable because it’s a perfect crystal. However, when you examine this material, one would never confuse it with a perfect crystal,” says Michael Strano, the Carbon P. Dubbs Professor of Chemical Engineering at MIT.
Third-generation solar cell technology is advancing rapidly. An engineering research team at The Hong Kong Polytechnic University (PolyU) has achieved a breakthrough in the field of perovskite/silicon tandem solar cells (TSCs), focusing on addressing challenges that include improving efficiency, stability and scalability.
The team has conducted a comprehensive analysis of TSC performance and provided strategic recommendations, which aim to raise the energy conversion efficiency of this new type of solar cell from the current maximum of approximately 34% to about 40%.
The team hopes to accelerate the commercialization of perovskite/silicon TSCs through industry-academia-research collaboration, while aligning with the nation’s strategic plan of carbon peaking and neutrality and promoting the development of innovative technologies such as artificial intelligence through renewable energy.
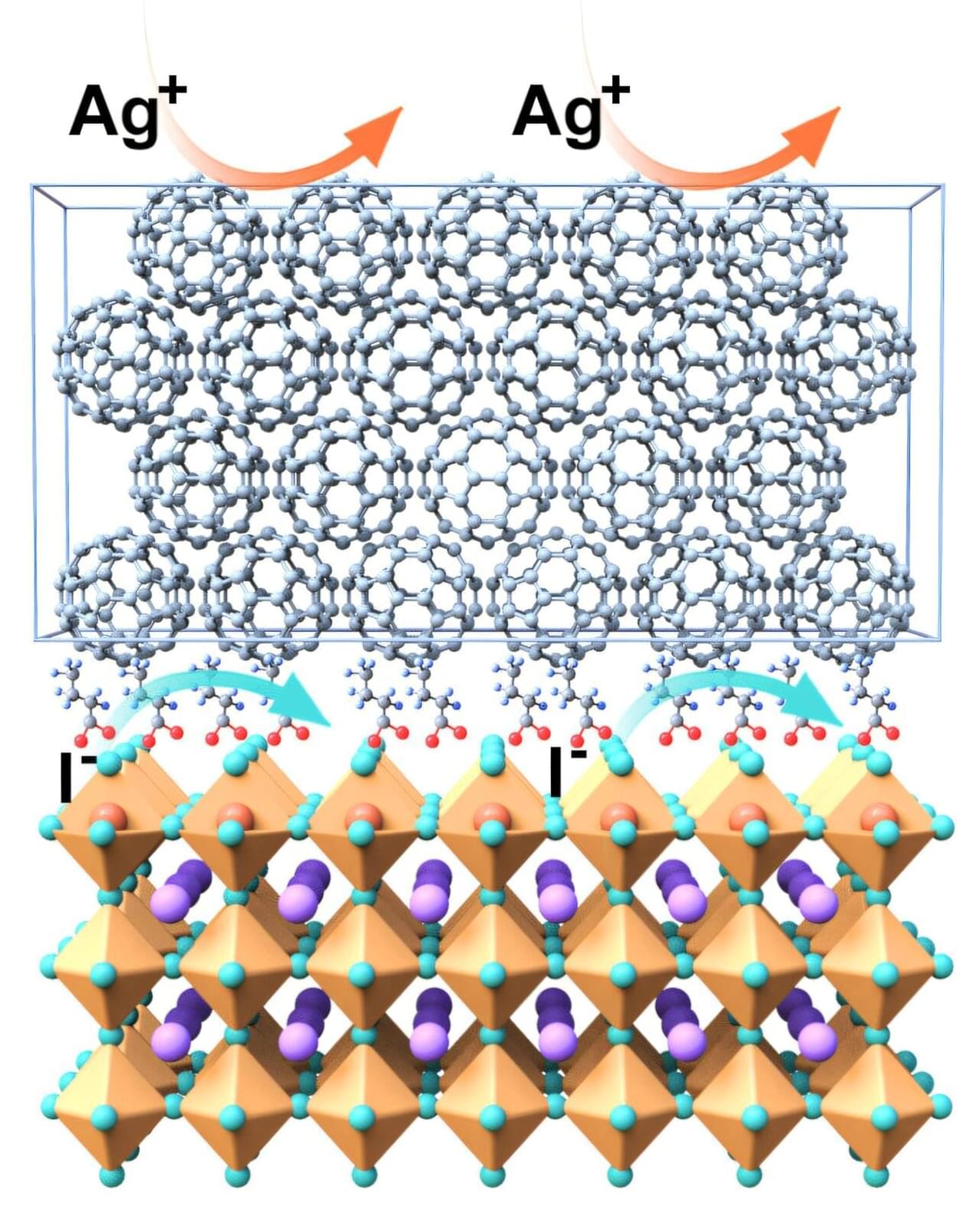
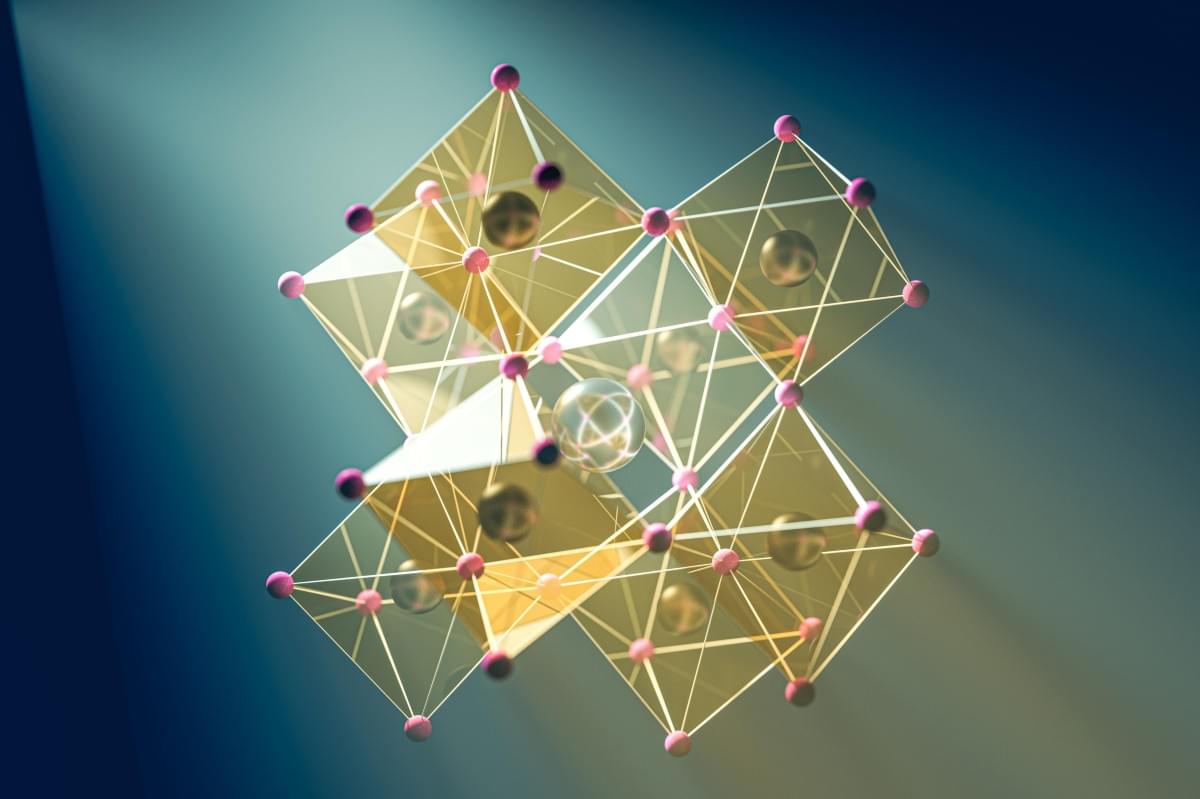
Perovskite solar cells are inexpensive to produce and generate a high amount of electric power per surface area. However, they are not yet stable enough, losing efficiency more rapidly than the silicon market standard. Now, an international team led by Prof. Dr. Antonio Abate has dramatically increased their stability by applying a novel coating to the interface between the surface of the perovskite and the top contact layer. This has even boosted efficiency to almost 27%, which represents the state-of-the-art.
After 1,200 hours of continuous operation under standard illumination, no decrease in efficiency was observed. The study involved research teams from China, Italy, Switzerland and Germany and has been published in Nature Photonics.
“We used a fluorinated compound that can slide between the perovskite and the buckyball (C60) contact layer, forming an almost compact monomolecular film,” explains Abate. These Teflon-like molecular layer chemically isolate the perovskite layer from the contact layer, resulting in fewer defects and losses. Additionally, the intermediate layer increases the structural stability of both adjacent layers, particularly the C60 layer, making it more uniform and compact.

Artificial intelligence (AI) is a foundational technology that could reshape our world, driving new scientific discoveries and helping us tackle humanity’s greatest challenges. Now, we’re asking where we can go to unlock its fullest potential.
The Sun is the ultimate energy source in our solar system, emitting more power than 100 trillion times humanity’s total electricity production. In the right orbit, a solar panel can be up to 8 times more productive than on earth, and produce power nearly continuously, reducing the need for batteries. In the future, space may be the best place to scale AI compute. Working backwards from there, our new research moonshot, Project Suncatcher, envisions compact constellations of solar-powered satellites, carrying Google TPUs and connected by free-space optical links. This approach would have tremendous potential for scale, and also minimizes impact on terrestrial resources.
We’re excited about this growing area of exploration, and our early research, shared today in “Towards a future space-based, highly scalable AI infrastructure system design,” a preprint paper, which describes our progress toward tackling the foundational challenges of this ambitious endeavor — including high-bandwidth communication between satellites, orbital dynamics, and radiation effects on computing. By focusing on a modular design of smaller, interconnected satellites, we are laying the groundwork for a highly scalable, future space-based AI infrastructure.