Penn Engineers have developed a novel design for solar-powered data centers that will orbit Earth and could realistically scale to meet the growing demand for AI computing while reducing the environmental impact of data centers.
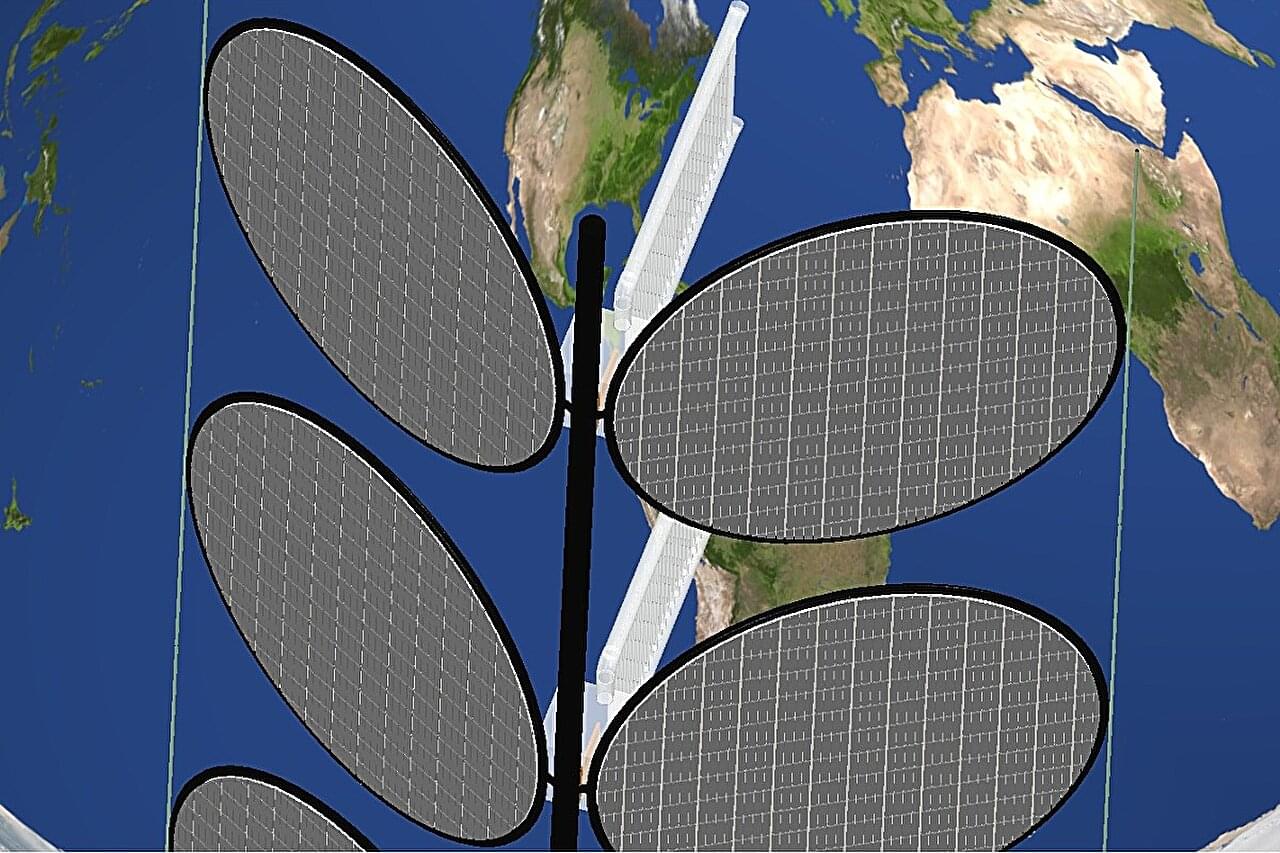
Reminiscent of a leafy plant, with multiple, hardware-containing stems connected to branching, leaf-like solar panels, the design leverages decades of research on “tethers,” rope-like cables that naturally orient themselves under the competing forces of gravity and centrifugal motion. This architecture could scale to the thousands of computing nodes needed to replicate the power of terrestrial data centers, at least for AI inference, the process of querying tools like ChatGPT after their training concludes.
Unlike prior designs, which typically require constant adjustments to keep solar panels pointed toward the sun, the new system is largely passive, its orientation maintained by natural forces acting on objects in orbit. By relying on these stabilizing effects, the design reduces weight, power consumption, and overall complexity, making large-scale deployment more feasible.