The AI pioneer on stepping down from Meta, the limits of large language models — and the launch of his new start-up



TRENDS Research & Advisory strives to present an insightful and informed view of global issues and challenges from a strategic perspective. Established in 2014 as an independent research center, TRENDS conducts specialized studies in the fields of international relations and political, economic and social sciences.


In the 21st century, new powerful technologies, such as different artificial intelligence (AI) agents, have become omnipresent and the center of public debate. With the increasing fear of AI agents replacing humans, there are discussions about whether individuals should strive to enhance themselves. For instance, the philosophical movement Transhumanism proposes the broad enhancement of human characteristics such as cognitive abilities, personality, and moral values (e.g., Grassie and Hansell 2011; Ranisch and Sorgner 2014). This enhancement should help humans to overcome their natural limitations and to keep up with powerful technologies that are increasingly present in today’s world (see Ranisch and Sorgner 2014). In the present article, we focus on one of the most frequently discussed forms of enhancement—the enhancement of human cognitive abilities.
Not only in science but also among the general population, cognitive enhancement, such as increasing one’s intelligence or working memory capacity, has been a frequently debated topic for many years (see Pauen 2019). Thus, a lot of psychological and neuroscientific research investigated different methods to increase cognitive abilities, but—so far—effective methods for cognitive enhancement are lacking (Jaušovec and Pahor 2017). Nevertheless, multiple different (and partly new) technologies that promise an enhancement of cognition are available to the general public. Transhumanists especially promote the application of brain stimulation techniques, smart drugs, or gene editing for cognitive enhancement (e.g., Bostrom and Sandberg 2009). Importantly, only little is known about the characteristics of individuals who would use such enhancement methods to improve their cognition. Thus, in the present study, we investigated different predictors of the acceptance of multiple widely-discussed enhancement methods. More specifically, we tested whether individuals’ psychometrically measured intelligence, self-estimated intelligence, implicit theories about intelligence, personality (Big Five and Dark Triad traits), and specific interests (science-fiction hobbyism) as well as values (purity norms) predict their acceptance of cognitive enhancement (i.e., whether they would use such methods to enhance their cognition).
https://www.jimruttshow.com/currents-ben-goertzel-2/
Jim talks with Ben Goertzel about the ideas in his recent essay “Three Viable Paths to True AGI.” They discuss the meaning of artificial general intelligence, Steve Wozniak’s basic AGI test, whether common tasks actually require AGI, a conversation with Joscha Bach, why deep neural nets are unsuited for human-level AGI, the challenge of extrapolating world-models, why imaginative improvisation might not be interesting to corporations, the 3 approaches that might have merit (cognition-level, brain-level, and chemistry-level), the OpenCog system Ben is working on, whether it’s a case of “good old-fashioned AI,” where evolution fits into the approach, why deep neural nets aren’t brain simulations & attempts to make them more realistic, a hypothesis about how to improve generalization, neural nets for music & the psychological landscape of AGI research, algorithmic chemistry & the origins of life problem, why AGI deserves more resources than it’s getting, why we may need better parallel architectures, how & how much society should invest in new approaches, the possibility of a cultural shift toward AGI viability, and much more.
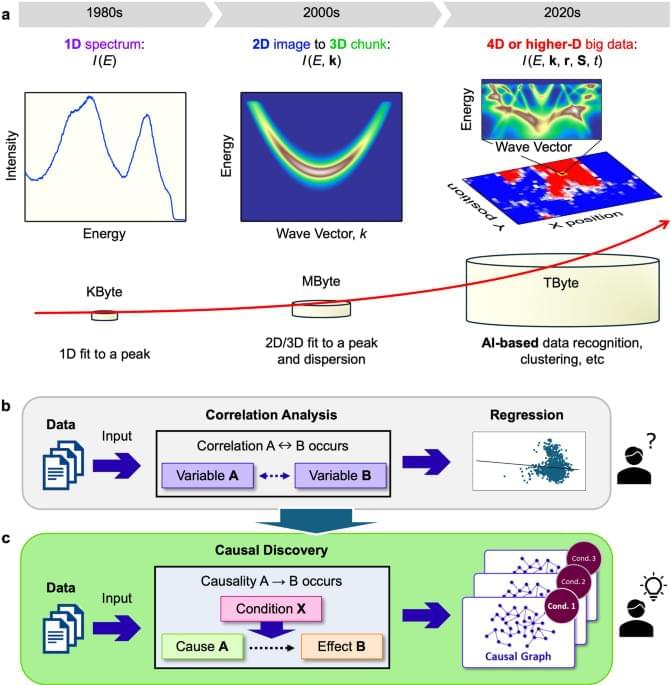
Scientific Reports volume 15, Article number: 43,291 (2025) Cite this article.
The platform can also hint at the type of respiratory issue involved, classifying cases as normal, obstructive, restrictive, or mixed. Obstructive patterns commonly appear in asthma and chronic obstructive pulmonary disease (COPD), while restrictive patterns are often linked to conditions such as pulmonary fibrosis.
The technology draws on the idea that cough sounds carry meaningful diagnostic clues. The researchers used a platform to classify the cases as ‘risk yes’ or ‘risk no’. When compared with physicians’ assessments, the model achieved a sensitivity of 97.27%. There was also strong agreement between the patterns identified by pulmonologists and the findings generated by the new tool.
Advances in AI have renewed interest in cough sound analysis as an accessible pre-screening method. Machine-learning models trained on large datasets can detect patterns associated with tuberculosis, Covid-19, asthma, and COPD, and can be built into portable devices or mobile apps for use in community settings.

A research team led by Professor Kanghyun Nam from the Department of Robotics and Mechanical Engineering at DGIST has developed a physical AI-based vehicle state estimation technology that accurately estimates the driving state of electric vehicles in real time.
This technology is viewed as a key advancement that can improve the core control performance of electric vehicles and greatly enhance the safety of autonomous vehicles. The work was conducted through international joint research with Shanghai Jiao Tong University in China and the University of Tokyo in Japan.
The work is published in the journal IEEE Transactions on Industrial Electronics.
