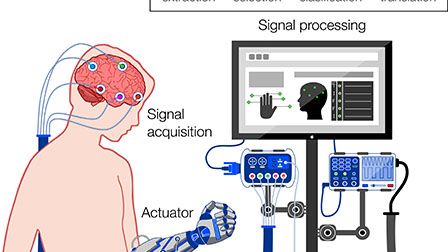
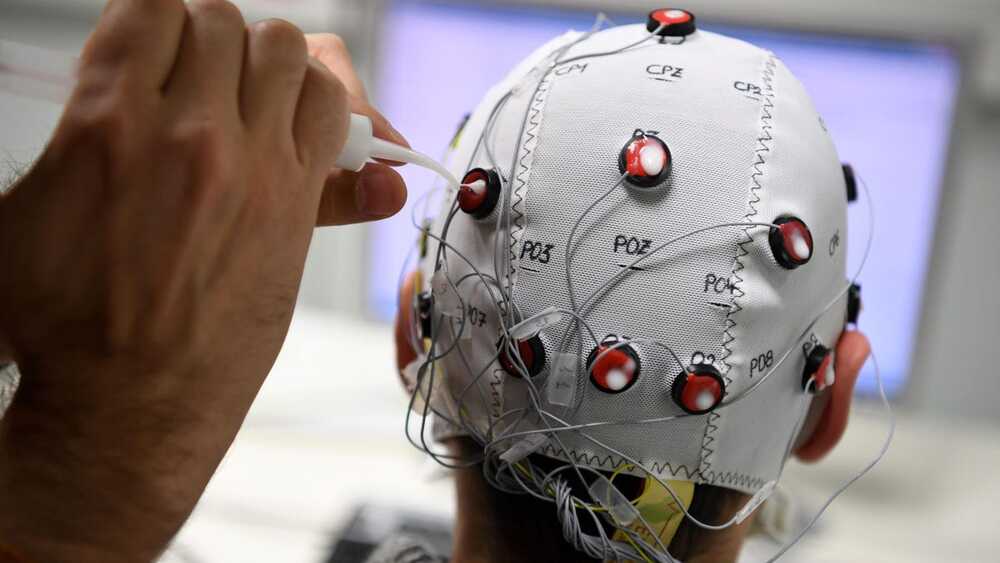
Brain–computer interfaces (BCIs) provide bidirectional communication between the brain and output devices that translate user intent into function. Among the different brain imaging techniques used to operate BCIs, electroencephalography (EEG) constitutes the preferred method of choice, owing to its relative low cost, ease of use, high temporal resolution, and noninvasiveness. In recent years, significant progress in wearable technologies and computational intelligence has greatly enhanced the performance and capabilities of EEG-based BCIs (eBCIs) and propelled their migration out of the laboratory and into real-world environments. This rapid translation constitutes a paradigm shift in human–machine interaction that will deeply transform different industries in the near future, including healthcare and wellbeing, entertainment, security, education, and marketing. In this contribution, the state-of-the-art in wearable biosensing is reviewed, focusing on the development of novel electrode interfaces for long term and noninvasive EEG monitoring. Commercially available EEG platforms are surveyed, and a comparative analysis is presented based on the benefits and limitations they provide for eBCI development. Emerging applications in neuroscientific research and future trends related to the widespread implementation of eBCIs for medical and nonmedical uses are discussed. Finally, a commentary on the ethical, social, and legal concerns associated with this increasingly ubiquitous technology is provided, as well as general recommendations to address key issues related to mainstream consumer adoption.