Australian and UK researchers grow brain cells in a lab that have learned to play a 1970s video game.


Scientists have transplanted human brain cells into the brains of baby rats, where the cells grew and formed connections.
It’s part of an effort to better study human brain development and diseases affecting this most complex of organs, which makes us who we are but has long been shrouded in mystery.
“Many disorders such as autism and schizophrenia are likely uniquely human” but “the human brain certainly has not been very accessible,” said said Dr. Sergiu Pasca, senior author of a study describing the work, published Wednesday in the journal Nature.



Summary: Brain cells grown in a petri dish can perform goal-directed tasks, such as learning to play a game of Pong.
Source: Cortical Labs.
A Melbourne-led team has for the first time shown that 800,000 brain cells living in a dish can perform goal-directed tasks – in this case the simple tennis-like computer game, Pong.

UW Medicine researchers have found that algorithms are as good as trained human evaluators at identifying red-flag language in text messages from people with serious mental illness. This opens a promising area of study that could help with psychiatry training and scarcity of care.
The findings were published in late September in the journal Psychiatric Services.
Text messages are increasingly part of mental health care and evaluation, but these remote psychiatric interventions can lack the emotional reference points that therapists use to navigate in-person conversations with patients.

The brain’s ability to adapt and rewire itself throughout life continues to surprise neuroscientists. Researchers have found a way to restore sight in adult mice with a form of congenital blindness, in spite of the rodents’ relative maturity.
The mice were modeling a rare human disorder of the eye’s retina, called leber congenital amaurosis (LCA), which often causes blindness or severe visual impairment at birth.
This inherited condition seems to be caused by a mutation in any one of dozens of genes associated with the retina and its light-sensing abilities.
Michael Levin is a developmental and synthetic biologist at Tufts University, where he is the Vannevar Bush Distinguished Professor of biology. He is a director of the Allen Discovery Center, director at the Tufts Center for Regenerative and Developmental Biology, and principal investigator at the Levin Lab.
0:00 intro.
1:38 bioelectricity and developmental biology.
7:56 memory and conditioning in GRNs.
11:50 is there a privileged cognitive substrate?
13:55 Godel type limits.
15:45 multi-scale competency architecture.
25:12 intelligence.
27:00 conceptual framework for cognition.
29:45 does cognition bottom out somewhere?
36:47 synthetic cognition.
39:23 sci-fi that captures this well.
45:16 consciousness, hard problem, and the consciousness of development.
51:09 where does the self come from?
54:06 how do different emergent levels interact.
56:50 top-down causality.
1:02:28 where do goals come from?
1:07:06 balancing conceptual and empirical work.
Michael Levin’s Website:
https://ase.tufts.edu/biology/labs/levin/
Podcast.
Spotify: https://open.spotify.com/show/0dUBLTl6qzOfA0xMndLFzq.
Google: https://www.google.com/podcasts?feed=aHR0cHM6Ly9mZWVkcy5yZWR…FhMw%3D%3D
Apple: https://podcasts.apple.com/us/podcast/thing-in-it-self/id1616881426
Amazon: https://music.amazon.ca/podcasts/9c6c08b2-e975-47d6-a897…in-it-self.
Social.
Twitter: https://twitter.com/thinginitself__
Instagram: https://www.instagram.com/thinginitself.pod/
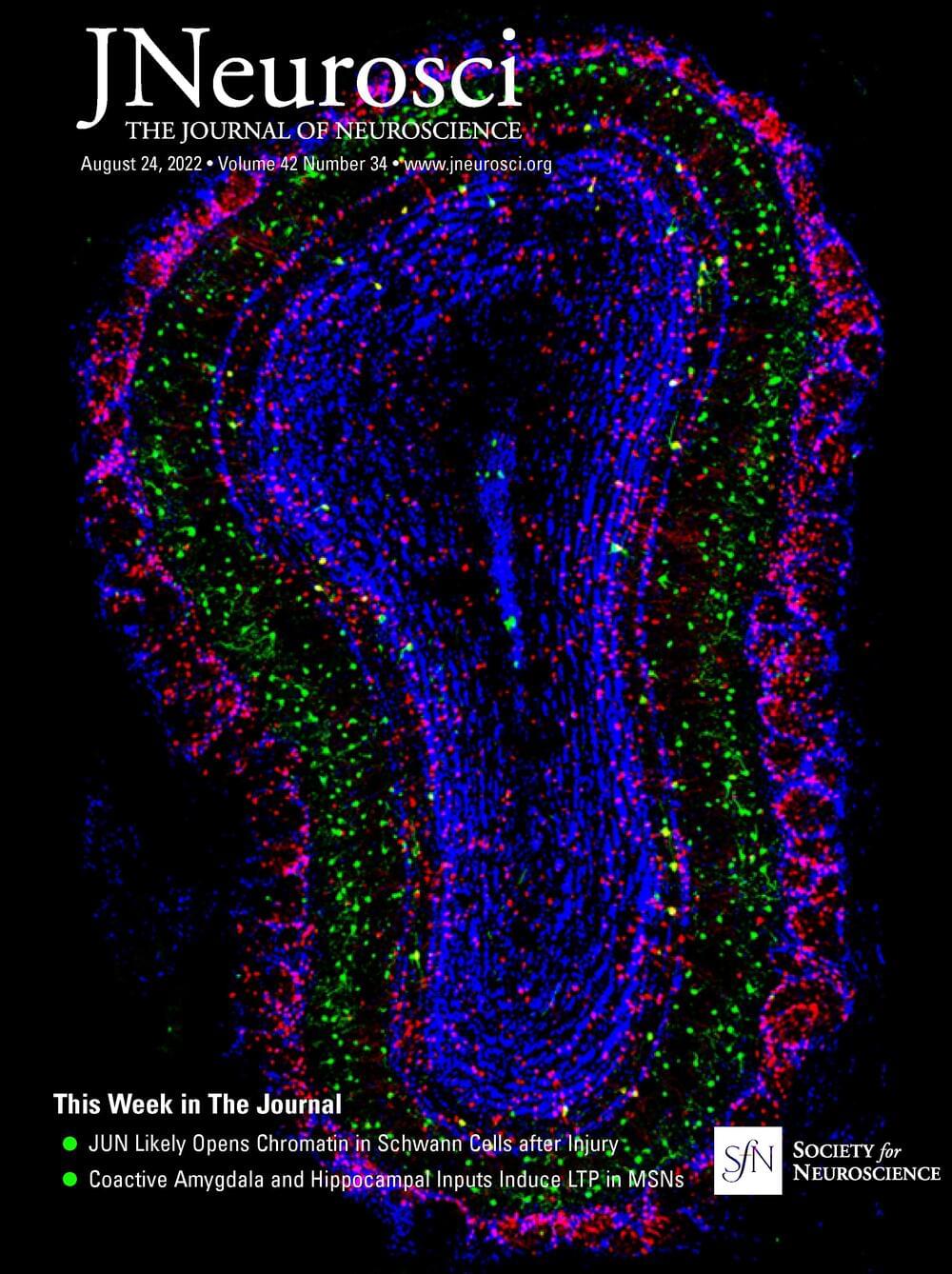
Large glutamatergic, somatic synapses mediate temporally precise information transfer. In the ventral nucleus of the lateral lemniscus, an auditory brainstem nucleus, the signal of an excitatory large somatic synapse is sign inverted to generate rapid feedforward inhibition with high temporal acuity at sound onsets, a mechanism involved in the suppression of spurious frequency information. The mechanisms of the synaptically driven input–output functions in the ventral nucleus of the lateral lemniscus are not fully resolved. Here, we show in Mongolian gerbils of both sexes that, for stimulation frequencies up to 200 Hz, the EPSC kinetics together with short-term plasticity allow for faithful transmission with only a small increase in latency. Glutamatergic currents are exclusively mediated by AMPARs and NMDARs. Short-term plasticity is frequency-dependent and composed of an initial facilitation followed by depression. Physiologically relevant output generation is limited by the decrease in synaptic conductance through short-term plasticity (STP). At this endbulb synapse, STP acts as a low pass filter and increases the dynamic range of the conductance dependent input–output relation, while NMDAR signaling slightly increases the sensitivity of the input–output function. Our computational model shows that STP-mediated filtering limits the intensity dependence of the spike output, thus maintaining selectivity to sound transients. Our results highlight the interaction of cellular features that together give rise to the computations in the circuit.
SIGNIFICANCE STATEMENT Auditory information processing in the brainstem is a prerequisite for generating our auditory representation of the environment. Thereby, many processing steps rely on temporally precise filtering. Precise feedforward inhibition is a key motif in auditory brainstem processing and produced through sign inversion at several large somatic excitatory synapses. A particular feature of the ventral nucleus of the lateral lemniscus is to produce temporally precise onset inhibition with little temporal variance independent of sound intensity. Our cell-physiology and modeling data explain how the synaptic characteristics of different current components and their short-term plasticity are tuned to establish sound intensity-invariant onset inhibition that is crucial for filtering out spurious frequency information.

Breakthrough AI programs can now generate videos from text input. The U.S. suicide rate and the prevalence of anxiety disorders are at all-time high. The White House has announced the “AI Bill of Rights.” What’s the connection between these 3 news items?
They all hint at how we will live our lives in the near future: As illusionists, making up imaginary worlds, fearing fabricated threats, led by conjurers, tricksters, and demagogues. For some, this prediction is already a good approximation of their present reality.
Let’s start with “AI,” the most exciting, confusing, and menacing technology of our times.
Are we already living in a “metaverse”?