In the ongoing work to realize the full potential of quantum computing, scientists could perhaps try peering into our own brains to see what’s possible: A new study suggests that the brain actually has a lot in common with a quantum computer.


In a study using specialized imaging techniques, Johns Hopkins Medicine researchers report distinctive changes in the “white matter” and other brain tissue physiology of those with post-treatment Lyme disease, a condition affecting 10% to 20% of the nearly half a million Americans who contract Lyme disease annually.
The study’s findings, published October 26 in the journal PLOS ONE, substantiate and help validate that memory difficulties and other cognitive difficulties experienced long-term by individuals with post-treatment Lyme disease are linked to functional and structural changes in the brain.
Lyme disease, whose early symptoms may include a characteristic rash, flu-like aches and fever, joint pain, and fatigue, is treated using a rigorous course of antibiotics, which usually clears the illness.

How does the human brain keep track of the order of events in a sequence?
Research suggests that ‘time cells’ – neurons in the hippocampus thought to represent temporal information – could be the glue that sticks our memories together in the right sequence so that we can properly recall the correct order in which things happened.
Evidence for these kinds of sequence-tracking time cells has previously been found in rats, where specific neuron assemblies are thought to support the recollection of events and the planning of action sequences – but for a long time, less was known about how episodic memory is encoded in the human brain.

Researchers conclude that one hemisphere of the brain can adequately function as if it were doing so for two hemispheres.
People who underwent surgery as children to remove half of their brain were still able to accurately recognize differences between pairs of words or faces.
The research was done to study brain plasticity and perception. Plasticity is when the brain can be molded to reorganize itself in the hemispheric region not injured, or in this case, the only hemispheric region that is there. The participants were able to correctly identify differences between words or faces with more than 80% accuracy.
The new design came with three fundamental improvements.
Researchers have finally managed to reduce the two-photon fluorescence microscope into a thumb size device that allows them to see inside the brain of live and active animals. The device called Mini2P weighs just 2.4 grams and can be attached to a mouse’s head without compromising its natural movements.
The microscope can record live images of neural landscapes, the likes of which have never been seen before. The innovation “opens the door to lines of scientific inquiry that were difficult, if not impossible, to initiate,” says Denise Cai, a neuroscientist at the Icahn School of Medicine at Mount Sinai in New York City. feat was achieved by Edvard Moser, professor of Psychology and Neuroscience at the Kavli Institute for Systems Neuroscience, together with Weijing Zong, a biological engineer and neuroscientist at the Moser Group.
Turtles, unlike humans, do not continue to age once their bodies reach adulthood because they are “negligibly senescent.” It is theoretically possible for them to live indefinitely, although it is unlikely to happen in actuality. They will eventually die of injury, predation, or sickness. It has been documented that tortoises and their cousins, turtles, can live for up to two hundred years without showing any signs of aging. A turtle that is a hundred years old can experience the same feelings of youth as a tortoise that is thirty years old. This enviable trait may be found in both fish and amphibians. The idea of aging terrifies humans, and it is understandable why. Nobody wants to age slowly and painfully into a state of ill health and old age where death appears preferable to life. However, not everyone thinks this way. There are others who desire to live longer, perhaps even indefinitely. And while a life without aging might sound like something that could only be found in the pages of a fantasy story, research in the field of science suggests that this possibility is very much within our reach.
In today’s video we look at Live until 200 YRS OLD!! Scientific cures for “The Aging Disease!” ~ Healthicity…Keep watching to see aging, the ageing, the healthy aging, is an aging expert, is aging slower, and reverse aging, fighting aging, how to fight aging, anti aging, aging wired, wired aging, aging matters, aging questions, how to stop aging, science of aging, ageing research, anti aging, aging tech support, slow aging, aging women, what is aging, allure aging, aging beauty, active aging, disrupt aging, aging support, aging science, decoding aging, future of aging, aging with grace.
Subscribe for Mental Health, Brain Health, and Psychology. Inspired by body hub, bestie, and BRIGHT SIDE
Inspired by the science of slowing down aging | WIRED
Inspired by Is Aging Reversible? A Scientific Look with David Sinclair | David Sinclair | TEDxBoston.
Researchers at the University of Texas at Austin have developed a decoder that uses information from fMRI scans to reconstruct human thoughts. Jerry Tang, Amanda LeBel, Shailee Jain and Alexander Huth have published a paper describing their work on the preprint server bioRxiv.
Prior efforts to create technology that can monitor brain waves and decode them to reconstruct a person’s thoughts have all consisted of probes placed in the brains of willing patients. And while such technology has proven useful for research efforts, it is not practical for use in other applications such as helping people who have lost the ability to speak. In this new effort, the researchers have expanded on work from prior studies by applying findings about reading and interpreting brain waves to data obtained from fMRI scans.
Recognizing that attempting to reconstruct brainwaves into individual words using fMRI was impractical, the researchers designed a decoding device that sought to gain an overall understanding of what was going on in the mind rather than a word-for-word decoding. The decoder they built was a computer algorithm that accepted fMRI data and returned paragraphs describing general thoughts. To train their algorithm, the researchers asked two men and one woman to lie in an fMRI machine while they listened to podcasts and recordings of people telling stories.
This is the first study to record such electrical activity from inside the brain.
How do people remember the things they’ve learned? To get to the bottom of the mystery, scientists undertook a study that looked deep inside the brain.
Neuroscientists from Northwestern University and clinicians from the University of Chicago Epilepsy Center examined the electrical activity in the brains of five patients at the center in response to sounds administered by the research team as part of a learning exercise.
Department of neurological surgery, the university of chicago.
To get to the bottom of the mystery, scientists undertook a study that looked deep inside the brain, where “previous learning was reactivated during sleep,” resulting in a refined memory.
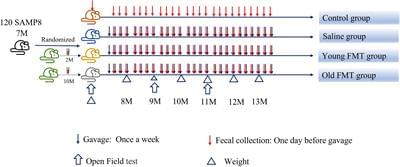
Recent evidence points out the role of the gut microbiota in the aging process. However, the specific changes and relevant interventions remain unclear. In this study, Senescence Accelerated Mouse-Prone 8 (SAMP8) mice were divided into four groups; young-FMT-group transplanted fecal microbiota from young donors (2–3°months old) and old-FMT-group transplanted from old donors (10–11°months old); additionally, other two groups either adult mice injected with saline solution or untreated mice served as the saline and blank control groups, respectively. All mice were intervened from their 7-months-old until 13-months-old. The open field test at 9 and 11°months of age showed that the mice transplanted with gut microbiota from young donors had significantly better locomotor and exploration ability than those of transplanted with old-donors gut microbiota and those of saline control while was comparable with the blank control. 16S rRNA gene sequencing showed that the gut microbiome of recipient mice of young donors was altered at 11°months of age, whereas the alternation of the gut microbiome of old-donor recipient mice was at 9°months. For comparison, the recipient mice in the blank and saline control groups exhibited changes in the gut microbiome at 10°months of age. The hallmark of aging-related gut microbiome change was an increase in the relative abundance of Akkermansia, which was significantly higher in the recipients transplanted with feces from older donors than younger donors at 9°months of age. This study shows that fecal microbiota transplantation from younger donors can delay aging-related declines in locomotor and exploration ability in mice by changing the gut microbiome.
Aging is inherently accompanied by the decline of physical and mental abilities, including locomotor, cognition, and bodily functions, to subsequently cause frailty syndrome, neurodegenerative diseases, and other age-related diseases, which reduce the quality of life of the aging population (Hou et al., 2019). Aging mechanisms and anti-aging interventions have long been a major focus of biomedical research, which is particularly relevant given the rapidly aging society.
The gut is a major organ for nutrients absorption, metabolism, and immunity, and contains hundreds of millions of microorganisms and their metabolites, which comprise the gut microbiota (Heintz and Mair, 2014) that interacts with host cells and tissues (Huang et al., 2021). Our previous study reported continuous changes in the gut microbiome of centenarians during their transition from a healthy status to death. The most significant changes of gut microbial communities in the period were found to occur at 7°months prior to death, suggesting that this may be a turning point of significant changes in the gut microbiome of centenarians (Luan et al., 2020). Recent studies have revealed an important relationship between the gut microbiome and aging-related diseases such as Alzheimer disease (Ticinesi et al., 2018; Haran and McCormick, 2021), suggesting that the gut microbiome plays an essential role in the aging process.