Cognitive scientist and philosopher, Professor Daniel Dennett, from Tufts University, takes us on a tour of the mind explaining why consciousness itself is a…
Category: neuroscience – Page 625
Daniel Dennett: Consciousness is a User Illusion (User Interface)
August 6, 2021
Daniel Dennett on Consciousness, Virtual Immortality, and Panpsychism | Closer To Truth Chats.
What is Panpsychism? | Rupert Sheldrake, Donald Hoffman, Phillip Goff, James Ladyman
What is panpsychism? Does it finally offer an explanation of consciousness? From the problems with materialism to the tradition of dualism, we asked the world’s leading thinkers to explain all.
#panpsychism #consciousness #reality #philosophy #mind.
Although one of the oldest philosophical theories, panpsychism is often seen as an outsider in the philosophy of mind. Recent interest in the hard problem of consciousness however has revived interest in panpsychism and mean it could provide new understandings of the mind and view of reality. Parapsychology expert Rupert Sheldrake, professor of philosophy Phillip Goff, cognitive psychologist Donald Hoffman, philosopher James Ladyman and professor of religion Mary-Jane Rubenstein give us their views.
For more on panpsychism and conciousness watch:
Understanding Conciousness | Full Debate | Rupert Sheldrake, George Ellis, Amie Thomasson.
https://www.youtube.com/watch?v=c-QOlTD0YHo.
Neuroscience of Conciousness | Raymond Tallis, Markus Gabriel, Susana Martinez Conde.
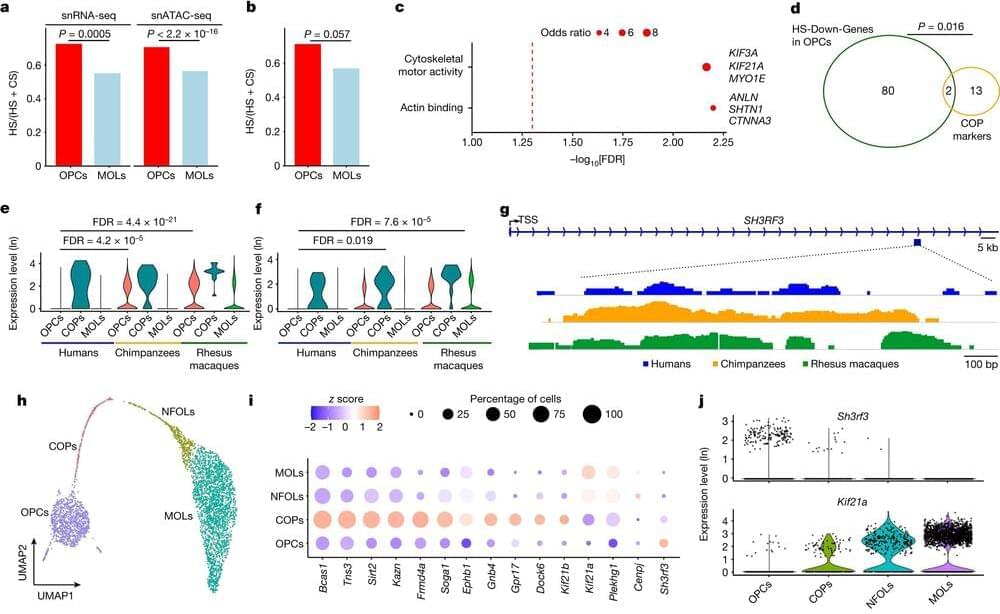
Study identifies characteristics specific to human brains
Researchers led by a team at UT Southwestern Medical Center have identified cellular and molecular features of the brain that set modern humans apart from their closest primate relatives and ancient human ancestors. The findings, published in Nature, offer new insights into human brain evolution.
“Most evolutionary studies on the human brain have focused on neurons because this cell type was thought to be responsible for our intelligence and enhanced cognitive abilities. This study gives us a renewed appreciation for other cells involved in brain function and the role they have played both in advancing cognition and our susceptibility to a number of cognitive diseases,” said study leader Genevieve Konopka, Ph.D., Professor of Neuroscience and a member of the Peter O’Donnell Jr. Brain Institute at UT Southwestern.
Since ancient times, people have been curious about what gives humans abilities that other animals don’t have, such as speech and language, Dr. Konopka explained. A range of previous studies have sought to answer this question by examining brain anatomy or performing genetic or molecular studies on whole brains or sections, experiments that provide a view of thousands of cells at a time.
Michael Levin | Cell Intelligence in Physiological and Morphological Spaces
Talk kindly contributed by Michael Levin in SEMF’s 2022 Spacious Spatiality.
https://semf.org.es/spatiality.
TALK ABSTRACT
Life was solving problems in metabolic, genetic, physiological, and anatomical spaces long before brains and nervous systems appeared. In this talk, I will describe remarkable capabilities of cell groups as they create, repair, and remodel complex anatomies. Anatomical homeostasis reveals that groups of cells are collective intelligences; their cognitive medium is the same as that of the human mind: electrical signals propagating in cell networks. I will explain non-neural bioelectricity and the tools we use to track the basal cognition of cells and tissues and control their function for applications in regenerative medicine. I will conclude with a discussion of our framework based on evolutionary scaling of intelligence by pivoting conserved mechanisms that allow agents, whether designed or evolved, to navigate complex problem spaces.
TALK MATERIALS
· The Electrical Blueprints that Orchestrate Life (TED Talk): https://www.ted.com/talks/michael_levin_the_electrical_bluep…trate_life.
· Michael Levin’s interviews and presentations: https://ase.tufts.edu/biology/labs/levin/presentations/
· Michael Levin’s publications: https://ase.tufts.edu/biology/labs/levin/publications/
· The Institute for Computationally Designed Organisms (ICDO): https://icdorgs.org/
MICHAEL LEVIN
Department of Biology, Tufts University: https://as.tufts.edu/biology.
Tufts University profile: https://ase.tufts.edu/biology/labs/levin/
Wyss Institute profile: https://wyss.harvard.edu/team/associate-faculty/michael-levin-ph-d/
Wikipedia: https://en.wikipedia.org/wiki/Michael_Levin_(biologist)
Google Scholar: https://scholar.google.com/citations?user=luouyakAAAAJ
Twitter: https://twitter.com/drmichaellevin.
LinkedIn: https://www.linkedin.com/in/michael-levin-b0983a6/
SEMF NETWORKS

Scientists reveal top five ‘anti-ageing’ foods that help add years to your life
WE all know we should be eating more healthily.
Improving your diet lowers your risk of several diseases, boosts immunity and supports brain development.
And now, new research has found incorporating a certain five foods into your meals could help you live longer — and they all happen to be plant based.

New Biomarkers Improve Diagnostics for Multiple Sclerosis & An MS-Like Disorder
Multiple sclerosis (MS) can cause a huge range of symptoms in different patients, and the severity can vary dramatically. It is an inflammatory condition in which the body attacks myelin sheaths, a protective insulation surrounding nerve cells. This can cause fatigue, pain, paralysis, and symptoms that gradually get worse. MS can be very difficult to diagnose because the symptoms can be so different in different patients, and the presence of brain lesions is the clearest indication of the disease. MRIs that can reveal those brain lesions are only useful once the disease have progressed to the point of brain damage, however.
The innate immune system presents a potential option for monitoring the progression of MS. The disease causes inflammation, so researchers tracked immune cells in the brain called macrophages, and assessed brain inflammation in a mouse model of MS. The findings have been reported in Science Translational Medicine.

Does cannabis use modify the effect of post-traumatic stress disorder on severe depression and suicidal ideation? Evidence from a population-based cross-sectional study of Canadians
Often seen negatively cannabis seen truthfully is a miracle plant even derivatives like cbd have cured some of the hardest mental disorders to cure like dementia alleviating symptoms to bring a person closer to health than before. Also now with this study veterans often seen incurable due to the unknown factors of ptsd and unknown factors of the human brain now are seeing relief through cannabis usage in a therapeutic setting 😀 Even the plant itself is some sorta miracle plant alleviating some thought incurable diseases.