Astrocytic lipid metabolism in brain signaling.
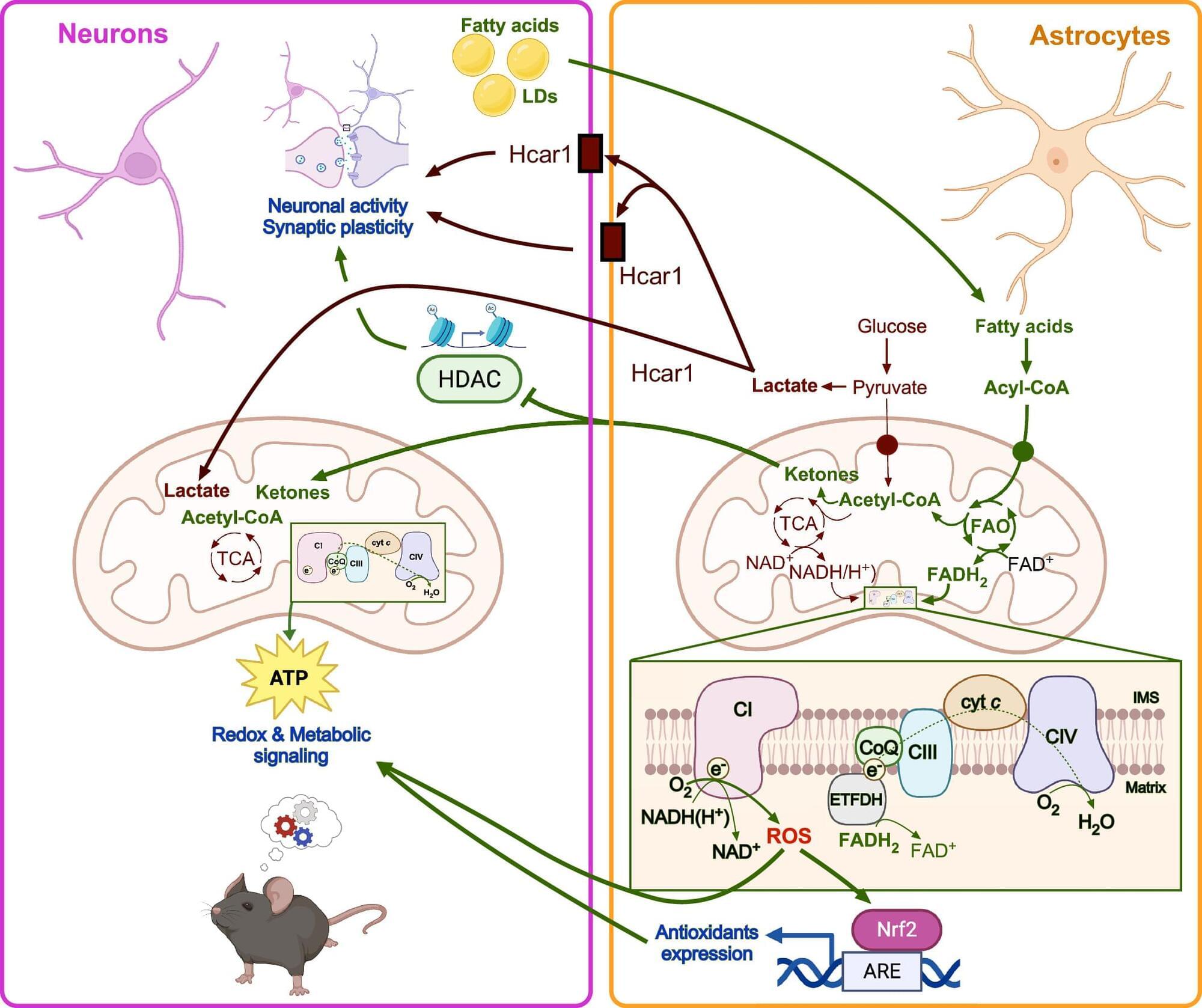
Glia previously thought to be support cells of brain but recent evidence suggest that the astrocytes, the most abundant glial cell type in addition to supplying neurons with lactate via glycolysis also actively engage in lipid metabolism, especially mitochondrial fatty acid β-oxidation.
Researchers in this review integrate astrocytic fatty acid ß-oxidation and ketogenesis, alongside other metabolic pathways converging on reactive oxygen species dynamics, including cholesterol metabolism and peroxisomal β-oxidation.
Thus, convergence of energy metabolism to signaling may provide new insights to central nervous system function and dysfunction. https://sciencemission.com/astrocytic-lipid-metabolism
Astrocytes, the most abundant glial cell type in the central nervous system, have traditionally been viewed from the perspective of metabolic support, particularly supplying neurons with lactate via glycolysis. This view has focused heavily on glucose metabolism as the primary mode of sustaining neuronal function. However, recent research challenges this paradigm by positioning astrocytes as dynamic metabolic hubs that actively engage in lipid metabolism, especially mitochondrial fatty acid β-oxidation. Far from serving solely as an energy source, fatty acid ß-oxidation in astrocytes orchestrates reactive oxygen species-mediated signaling pathways that modulate neuron-glia communication and cognitive outcomes.