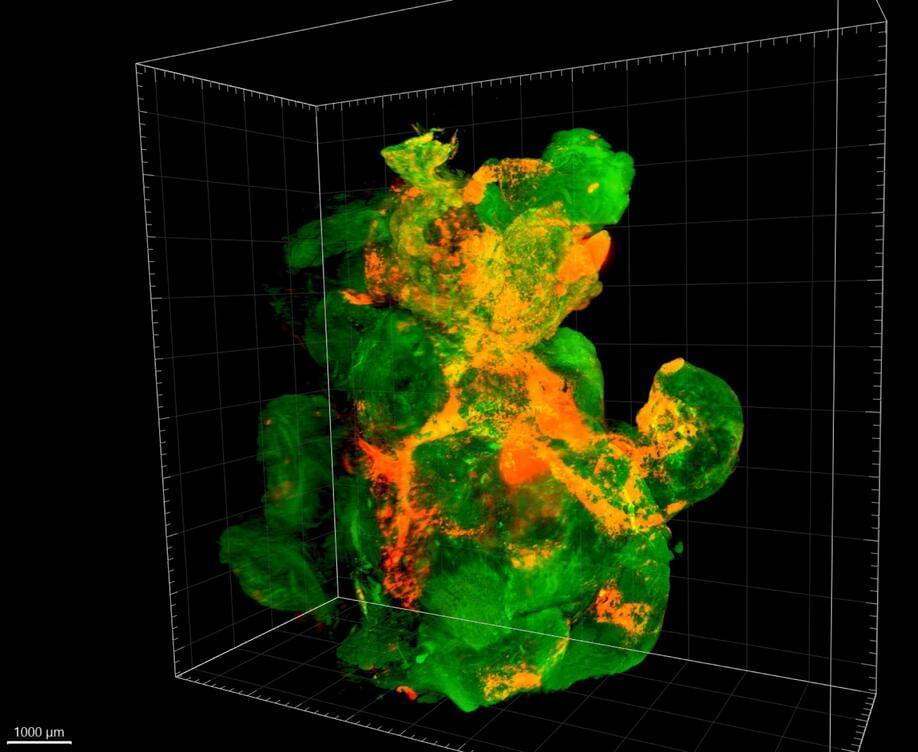
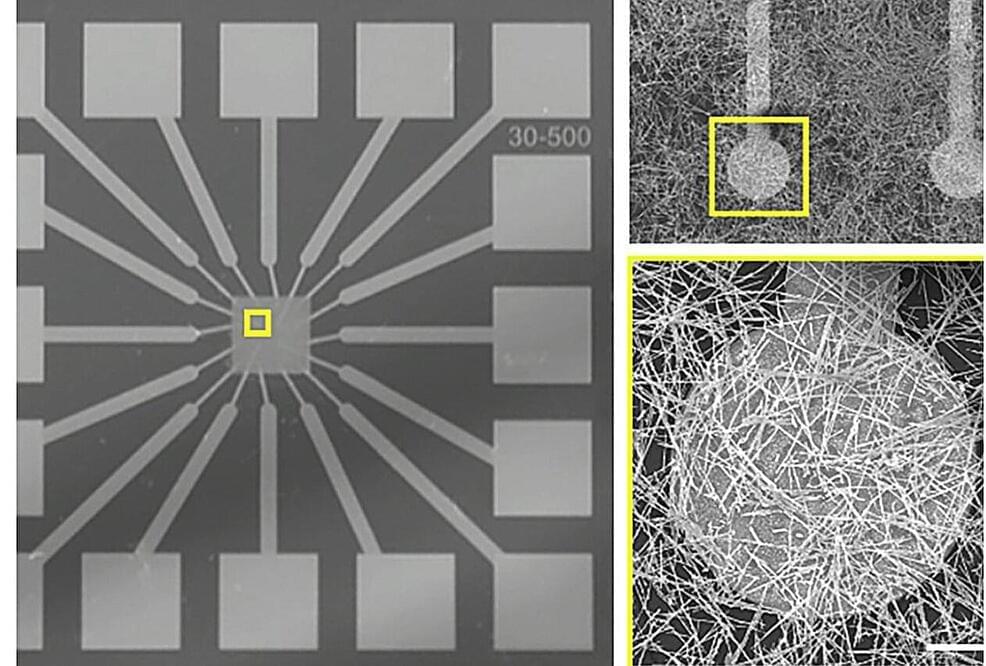
The human mind is by far one of the most amazing natural phenomena known to man. It embodies our perception of reality, and is in that respect the ultimate observer. The past century produced monumental discoveries regarding the nature of nerve cells, the anatomical connections between nerve cells, the electrophysiological properties of nerve cells, and the molecular biology of nervous tissue. What remains to be uncovered is that essential something – the fundamental dynamic mechanism by which all these well understood biophysical elements combine to form a mental state. In this chapter, we further develop the concept of an intraneuronal matrix as the basis for autonomous, self–organized neural computing, bearing in mind that at this stage such models are speculative. The intraneuronal matrix – composed of microtubules, actin filaments, and cross–linking, adaptor, and scaffolding proteins – is envisioned to be an intraneuronal computational network, which operates in conjunction with traditional neural membrane computational mechanisms to provide vastly enhanced computational power to individual neurons as well as to larger neural networks. Both classical and quantum mechanical physical principles may contribute to the ability of these matrices of cytoskeletal proteins to perform computations that regulate synaptic efficacy and neural response. A scientifically plausible route for controlling synaptic efficacy is through the regulation of neural transport of synaptic proteins and of mRNA. Operations within the matrix of cytoskeletal proteins that have applications to learning, memory, perception, and consciousness, and conceptual models implementing classical and quantum mechanical physics are discussed. Nanoneuroscience methods are emerging that are capable of testing aspects of these conceptual models, both theoretically and experimentally. Incorporating intra–neuronal biophysical operations into existing theoretical frameworks of single neuron and neural network function stands to enhance existing models of neurocognition.