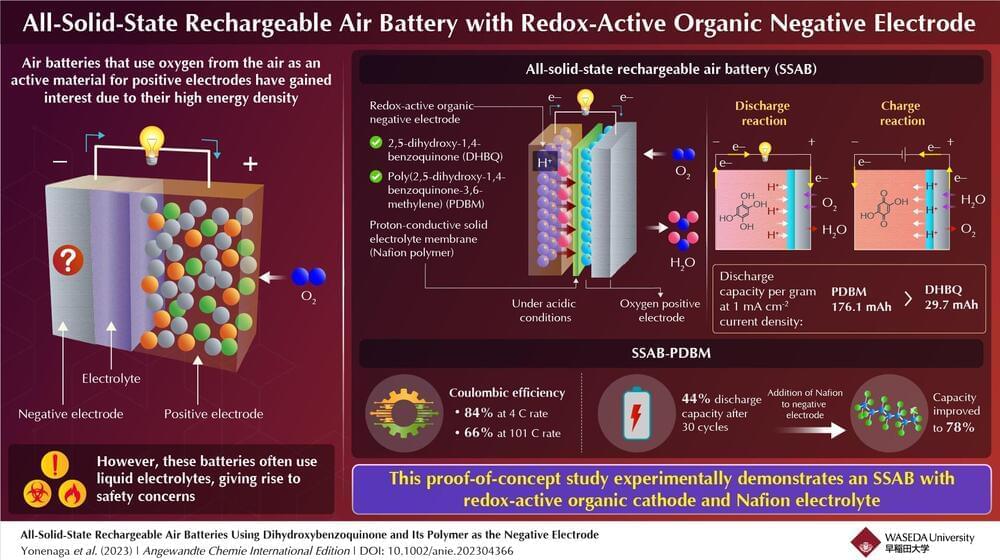
Metals are typically used as active materials for negative electrodes in batteries. Recently, redox-active organic molecules, such as quinone-and amine-based molecules, have been used as negative electrodes in rechargeable metal–air batteries with oxygen-reducing positive electrodes. Here, protons and hydroxide ions participate in the redox reactions. Such batteries exhibit high performance, close to the maximum capacity that is theoretically possible.
Furthermore, using redox-active organic molecules in rechargeable air batteries overcomes problems associated with metals, including the formation of structures called ‘dendrites,’ which impact battery performance, and have negative environmental impact. However, these batteries use liquid electrolytes—just like metal-based batteries—which pose major safety concerns like high electrical resistance, leaching effects, and flammability.
Now, in a recent study published in Angewandte Chemie International Edition, a group of Japanese researchers have developed an all-solid-state rechargeable air battery (SSAB) and investigated its capacity and durability. The study was led by Professor Kenji Miyatake from Waseda University and the University of Yamanashi, and co-authored by Professor Kenichi Oyaizu from Waseda University.