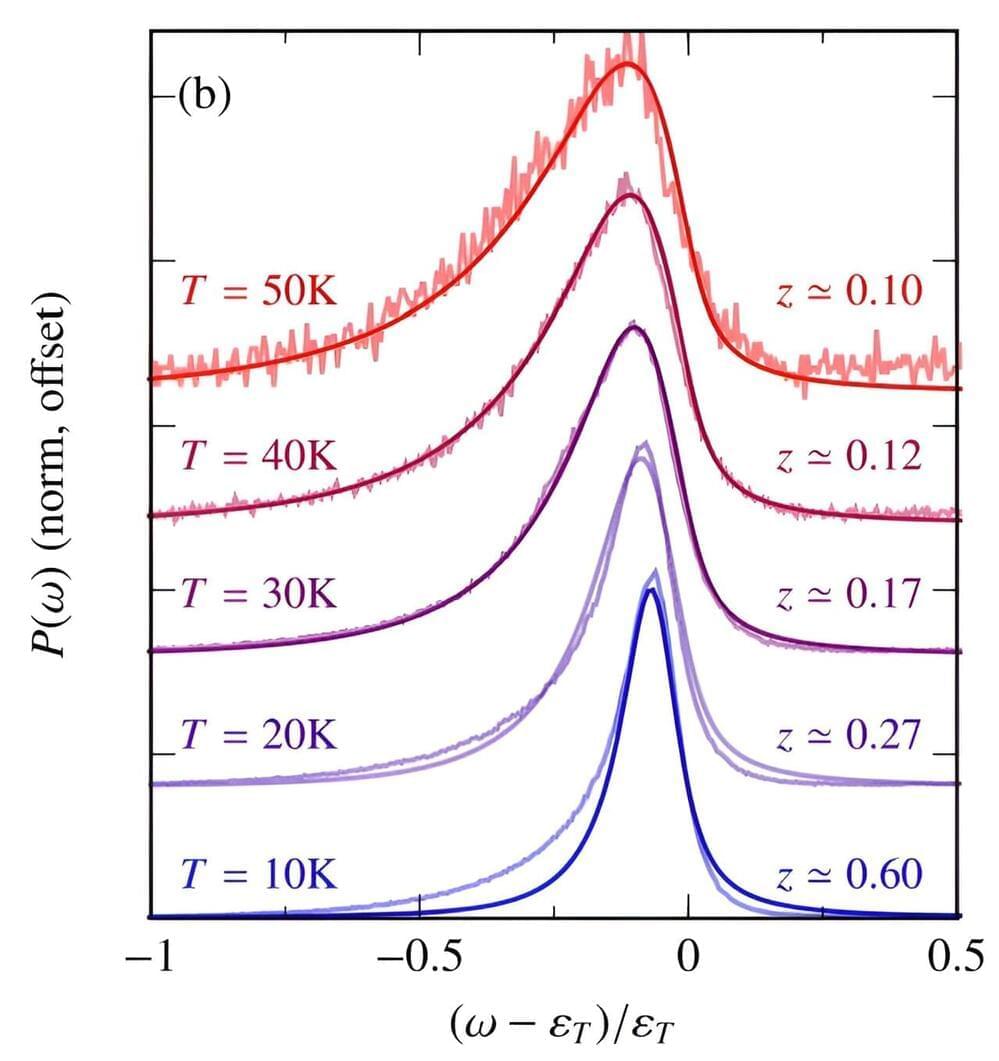
Despite its waif-like proportions, scientists have found over the years that graphene is exceptionally strong. And when the material is stacked and twisted in specific contortions, it can take on surprising electronic behavior.
Now, MIT physicists have discovered another surprising property in graphene: When stacked in five layers, in a rhombohedral pattern, graphene takes on a very rare, “multiferroic” state, in which the material exhibits both unconventional magnetism and an exotic type of electronic behavior, which the team has coined ferro-valleytricity.