A new theory has finally deciphered the physical mechanisms of fracture in soft materials. This discovery could soon lead to new, defect-free materials that are more resistant and durable as well as environmentally friendly. The article “Elastic instability behind brittle fracture” was recently published by Physical Review Letters.
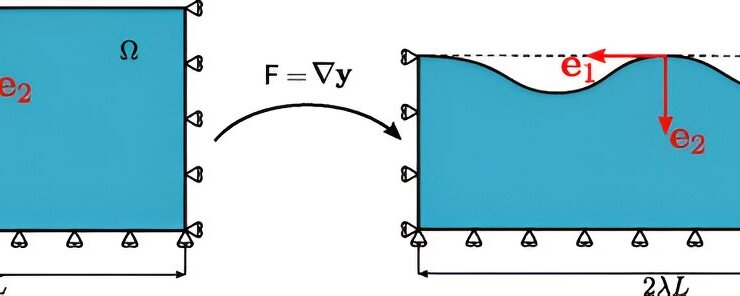
“We have revealed that fracture propagates from the free surface of the material, starting from an elastic instability that breaks the symmetry of the object. Then, the rupture drastically extends with an intricate network of cracks spreading like a turbulence phenomenon similar to what we observe in fluids, like during vortex formation,” explains Pasquale Ciarletta from the MOX Laboratory, Department of Mathematics at Politecnico di Milano.
This discovery stimulates significant applications in various technological sectors. For instance, in the production of micro and nano devices, where materials need to be extremely resistant and defect-free.