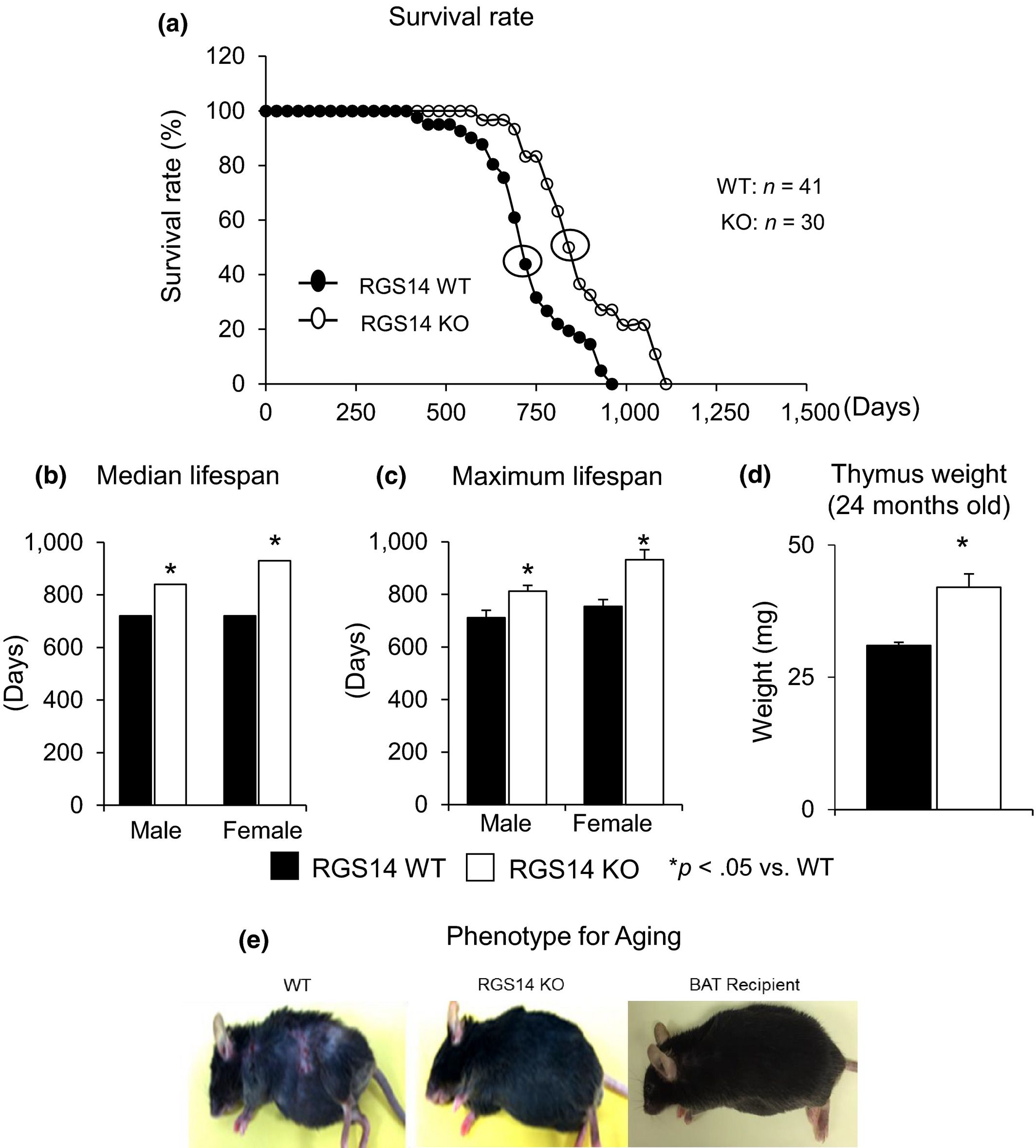
Disruption of the regulator for G protein signaling 14 (RGS14) knockout (KO) in mice extends their lifespan and has multiple beneficial effects related to healthful aging, that is, protection from obesity, as reflected by reduced white adipose tissue, protection against cold exposure, and improved metabolism. The observed beneficial effects were mediated by improved mitochondrial function. But most importantly, the main mechanism responsible for the salutary properties of the RGS14 KO involved an increase in brown adipose tissue (BAT), which was confirmed by surgical BAT removal and transplantation to wild‐type (WT) mice, a surgical simulation of a molecular knockout. This technique reversed the phenotype of the RGS14 KO and WT, resulting in loss of the improved metabolism and protection against cold exposure in RGS14 KO and conferring this protection to the WT BAT recipients. Another mechanism mediating the salutary features in the RGS14 KO was increased SIRT3. This mechanism was confirmed in the RGS14 X SIRT3 double KO, which no longer demonstrated improved metabolism and protection against cold exposure. Loss of function of the Caenorhabditis elegans RGS‐14 homolog confirmed the evolutionary conservation of this mechanism. Thus, disruption of RGS14 is a model of healthful aging, as it not only enhances lifespan, but also protects against obesity and cold exposure and improves metabolism with a key mechanism of increased BAT, which, when removed, eliminates the features of healthful aging.