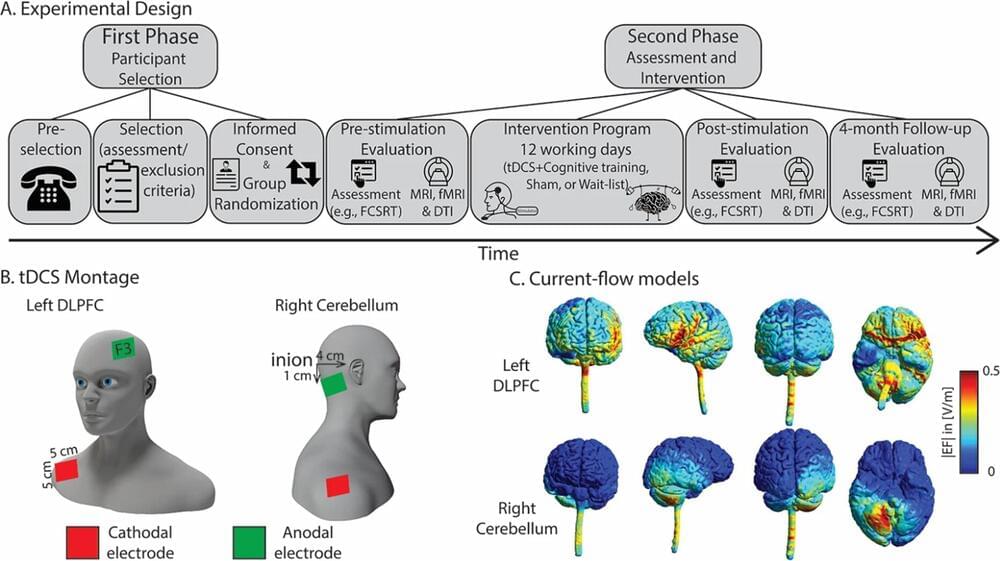
A recent study demonstrated that non-invasive stimulation of the right cerebellum led to improvements in episodic memory performance in healthy elderly individuals, at the end of a 12-day neurostimulation program, and also at the point of a 4-month follow-up.
The steady increase in average life expectancy poses significant challenges to individuals, families, and societies across multiple dimensions. Estimating that by 2050 one in every six individuals will be over the age of 65, the study of aging and its association with cognitive decline, neurodegenerative diseases and overall frailty is becoming increasingly important.
Therefore, it has been an important goals of neurosciences research to understand the relationship between the aging brain and episodic memory deficits and to develop interventions to mitigate the age-related decline in our ability to remember personal past events (episodic memory).