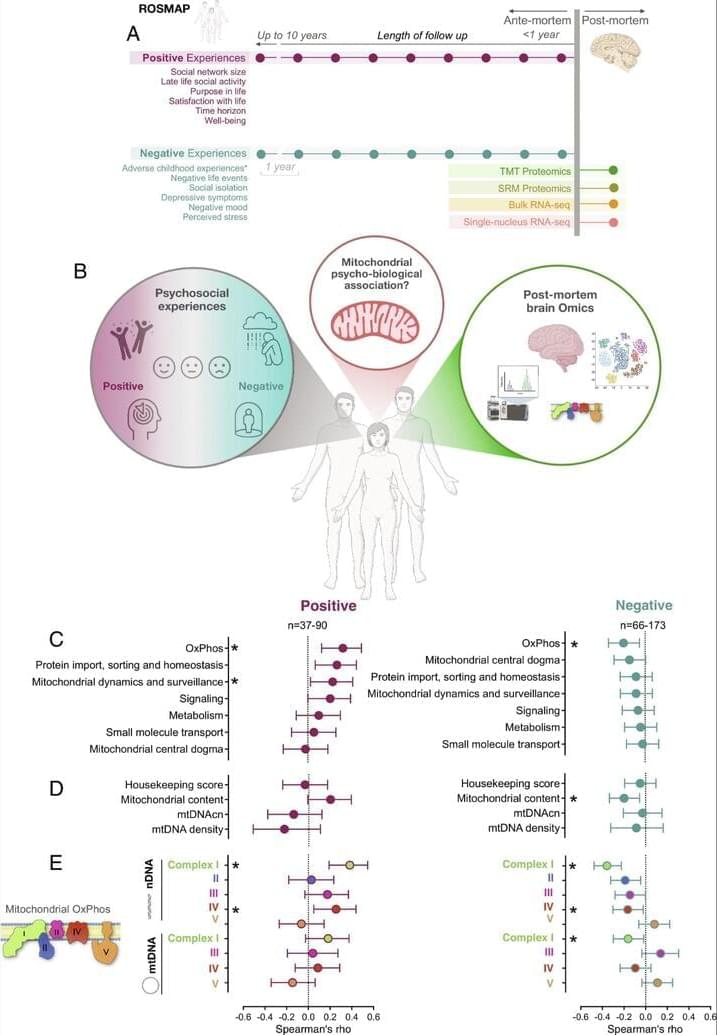
Most of our progress in disease treatment and prevention to date has been the product of the linear process of hit-or-miss efforts to find useful interventions. Because we have lacked tools for systematically exploring all possible treatments, discoveries under this paradigm have owed a lot to chance. Likely the most notable chance breakthrough in medicine was the accidental discovery of penicillin — which opened up the antibiotic revolution and has since saved perhaps as many as 200 million lives. But even when discoveries aren’t literally accidental, it still takes good fortune for researchers to achieve breakthroughs with traditional methods. Without the ability to exhaustively simulate possible drug molecules, researchers have to rely on high-throughput screening and other painstaking laboratory methods, which are much slower and more inefficient.
To be fair, this approach has brought great benefits. A thousand years ago, European life expectancy at birth was just in the twenties, since so many people died in infancy or youth from diseases like cholera and dysentery, which are now easily preventable. By the middle of the nineteenth century, life expectancy in the United Kingdom and the United States had increased to the forties. As of 2023, it has risen to over eighty in much of the developed world. So, we have nearly tripled life expectancy in the past thousand years and doubled it in the past two centuries. This was largely achieved by developing ways to avoid or kill external pathogens — bacteria and viruses that bring disease from outside our bodies.
Today, though, most of this low-hanging fruit has been picked. The remaining sources of disease and disability spring mostly from deep within our own bodies. As cells malfunction and tissues break down, we get conditions like cancer, atherosclerosis, diabetes, and Alzheimer’s. To an extent we can reduce these risks through lifestyle, diet, and supplementation — what I call the first bridge to radical life extension. But those can only delay the inevitable. This is why life expectancy gains in developed countries have slowed since roughly the middle of the twentieth century. For example, from 1,880 to 1900, life expectancy at birth in the United States increased from about thirty-nine to forty-nine, but from 1980 to 2000 — after the focus of medicine had shifted from infectious disease to chronic and degenerative disease — it only increased from seventy-four to seventy-six.