Researchers have discovered that DNA methylation is crucial for reprogramming astrocytes into stem cells in the adult mouse brain, especially after ischemic injury, with potential implications for regenerative medicine.


Some of our closest invertebrate cousins, like this Acorn worm, have the ability to perfectly regenerate any part of their body that’s cut off — including the head and nervous system. Humans have most of the same genes, so scientists are trying to work out whether human regeneration is possible, too.
Regeneration – now that’d be a nice superpower to have. Injure an arm? Chop it off and wait for it to grow back. Dicky knee? Ingrown toenail? Lop off your leg and get two for one!
It sounds ridiculous, but there’s a growing number of scientists that believe body part regeneration is not only possible, but achievable in humans. After all, not only are there plenty of animals that can do it, we can do it ourselves for our skin, nails, and bits of other organs.

COPENHAGEN, Denmark — In the never-ending quest to unlock the secrets of a long and healthy life, researchers at the University of Copenhagen have made a remarkable discovery. Their study has identified a specific gene that plays a crucial role in extending longevity across various species, including humans.
Publishing their work in the journal Cell Reports, researchers say the gene in question is called OSER1, and it encodes a protein that the team has dubbed a “novel pro-longevity factor.”
“We identified this protein that can extend longevity. It is a novel pro-longevity factor, and it is a protein that exists in various animals, such as fruit flies, nematodes, silkworms, and in humans,” says Professor Lene Juel Rasmussen, the senior author behind the study, in a media release.

Cellular senescence is a diverse phenotype characterised by permanent cell cycle arrest and an associated secretory phenotype (SASP) which includes inflammatory cytokines. Typically, senescent cells are removed by the immune system, but this process becomes dysregulated with age causing senescent cells to accumulate and induce chronic inflammatory signalling. Identifying senescent cells is challenging due to senescence phenotype heterogeneity, and senotherapy often requires a combinatorial approach. Here we systematically collected 119 transcriptomic datasets related to human fibroblasts, forming an online database describing the relevant variables for each study allowing users to filter for variables and genes of interest. Our own analysis of the database identified 28 genes significantly up-or downregulated across four senescence types (DNA damage induced senescence (DDIS), oncogene induced senescence (OIS), replicative senescence, and bystander induced senescence) compared to proliferating controls. We also found gene expression patterns of conventional senescence markers were highly specific and reliable for different senescence inducers, cell lines, and timepoints. Our comprehensive data supported several observations made in existing studies using single datasets, including stronger p53 signalling in DDIS compared to OIS. However, contrary to some early observations, both p16 and p21 mRNA levels rise quickly, depending on senescence type, and persist for at least 8–11 days. Additionally, little evidence was found to support an initial TGFβ-centric SASP. To support our transcriptomic analysis, we computationally modelled temporal protein changes of select core senescence proteins during DDIS and OIS, as well as perform knockdown interventions. We conclude that while universal biomarkers of senescence are difficult to identify, conventional senescence markers follow predictable profiles and construction of a framework for studying senescence could lead to more reproducible data and understanding of senescence heterogeneity.
Multiple studies now suggest that the accumulation of senescent cells is causal in ageing (Childs et al., 2015; Mylonas and O’Loghlen, 2022; van Deursen, 2014; Wlaschek et al., 2021), and their ablation extends healthspan and mean lifespan in rodents (Baker et al., 2016; Baker et al., 2011). Novel senolytic and senostatic drugs are in development (Kim and Kim, 2019; Niedernhofer and Robbins, 2018) with some drugs in clinical trials (Hickson et al., 2019; Justice et al., 2019) which might shortly lead to treatments capable of improving healthspan and extending lifespan in humans. However, the exact nature of senescent cells is often difficult to define, with multiple studies indicating that the most common biomarkers of senescence show different profiles across cell lines, types of senescence inducer, and the timepoint after the initial stimulus (Avelar et al., 2020; Basisty et al., 2020; Casella et al., 2019; Hernandez-Segura et al., 2017; Neri et al., 2021).
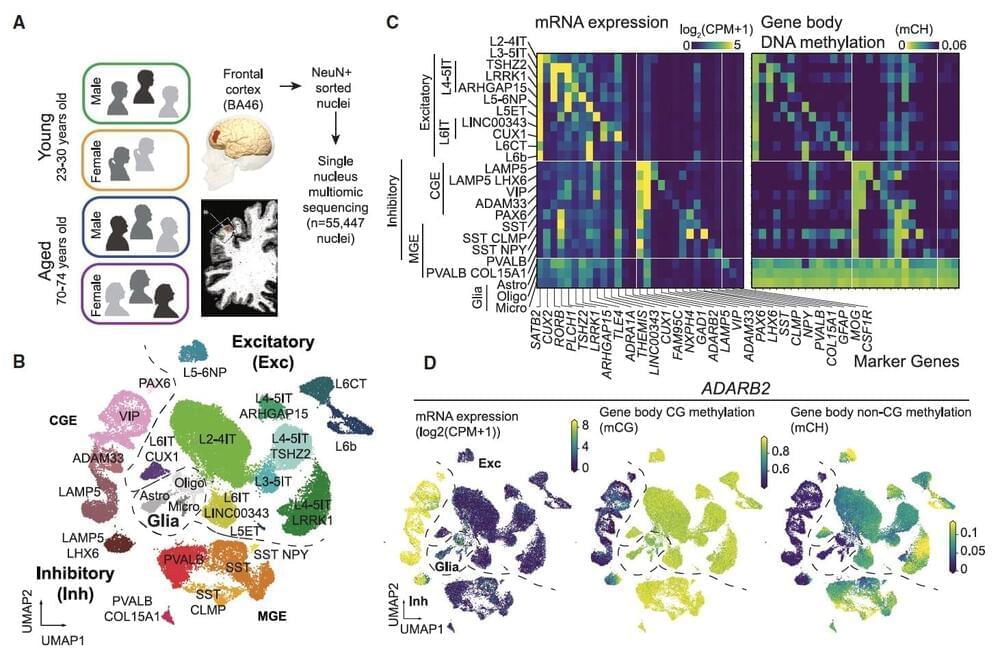
Aging is known to have profound effects on the human brain, prompting changes in the composition of cells and the expression of genes, while also altering aspects of the interaction between genes and environmental factors. While past neuroscience studies have pinpointed many of the molecular changes associated with aging, the age-related genetic factors influencing specific neuron populations remains poorly understood.
Recent studies on flies, mice, primates and human brain tissue utilizing single-cell or single-nucleus RNA-sequencing and genetic experimental techniques shed new light on these cell-type-specific changes. For instance, they unveiled the effects of aging on glial cells in the mouse and human brain, associations between cell-specific changes and modified chromatin proteins, and the influence of DNA methylation in the aging of various tissues.
Researchers at University of California (UC) San Diego and Salk Institute recently carried out a study aimed at better understanding how both age and sex impact human cortical neurons at a single-cell level. Their findings, published in Neuron, offer new insights into how aging affects cell composition, gene expression and DNA methylation across human brain cell types, while also uncovering differences between gene expression and DNA methylation in females and males.

Here the notorious but eloquent transhumanism critic Wesley J. Smith takes a swipe at the quickly growing movement to overcome death with science. New story in Merion West!
“Utopians often produce evil because their movement’s aspirations become paramount —that is, more important than avoiding acts ‘traditionally perceived as immoral.’ If enough people follow Istvan on the transhuman roller coaster, people could eventually get hurt.”
“I’m not afraid of dying. I just don’t want to be there when it happens.” – Woody Allen.
In 2016, transhumanism proselytizer Zoltan Istvan ran for president promising to defeat death while touring the country in a bus redesigned to look like a coffin. It was a great gimmick that made him, perhaps, the most famous transhumanist in the world.
I know and like Istvan. I admire his indefatigable work ethic that has him writing hundreds of transhumanist-boosting columns and engaging in countless interviews (including by opponents like me). But his recent piece in Merion West “When We’re Overly Optimistic about the Pace of Life Extension Research” took a dark and disturbing turn. He warns that at the current pace of life-extending research, the transhumanist goal of living indefinitely will not be attained during his lifetime (based on a formula he concocted he calls, “the senescence inference”). In 2,131, he moans, our expected lifespan will “only” be 165 years, and it will take “well over a millennium to attain Methuselah-like lifespans nearing 1,000 years.”

Researchers at The University of Texas MD Anderson Cancer Center have shown that therapeutically restoring ‘youthful’ levels of a specific subunit of the telomerase enzyme can significantly reduce the signs and symptoms of aging in preclinical models. If these findings are validated in clinical trials, they could have important therapeutic implications for age-related diseases such as Alzheimer’s, Parkinson’s, heart disease, and cancer.
The study, published in Cell, identified a small molecule compound that restores physiological levels of telomerase reverse transcriptase (TERT), which normally is repressed with the onset of aging. Maintenance of TERT levels in aged lab models reduced cellular senescence and tissue inflammation, spurred new neuron formation with improved memory, and enhanced neuromuscular function, which increased strength and coordination.
The researchers show that TERT functions not only to extend telomeres, but also acts as a transcription factor to affect the expression of many genes directing neurogenesis, learning and memory, cellular senescence, and inflammation.

Will artificial intelligence save us or kill us all? In Japan, AI-driven technology promises better lives for an aging population. But researchers in Silicon Valley are warning of untamable forces being unleashed– and even human extinction.
Will artificial intelligence make life better for humans or lead to our downfall? As developers race toward implementing AI in every aspect of our lives, it is already showing promise in areas like medicine. But what if it is used for nefarious purposes?
In Japan, the inventor and scientist behind the firm Cyberdyne is working to make life better for the sick and elderly. Professor Yoshiyuki Sankai’s robot suits are AI-driven exoskeletons used in rehabilitative medicine to help stroke victims and others learn to walk again. But he doesn’t see the benefits of AI ending there; he predicts a future world where AIs will live in harmony with humans as a new, benevolent species.
Yet in Silicon Valley, the cradle of AI development, there is an unsettling contradiction: a deep uncertainty among many developers about the untamable forces they are unleashing. Gabriel Mukobi is a computer science graduate student at Stanford who is sounding the alarm that AI could push us toward disaster– and even human extinction. He’s at the forefront of a tiny field of researchers swimming against the current to make sure AI is safe and beneficial for everyone.
What are the promises and perils of AI? And who gets to decide how it will be used?
#documentary #dwdocumentary #technology #AI #UsandThem.
Enjoy the videos and music you love, upload original content, and share it all with friends, family, and the world on YouTube.