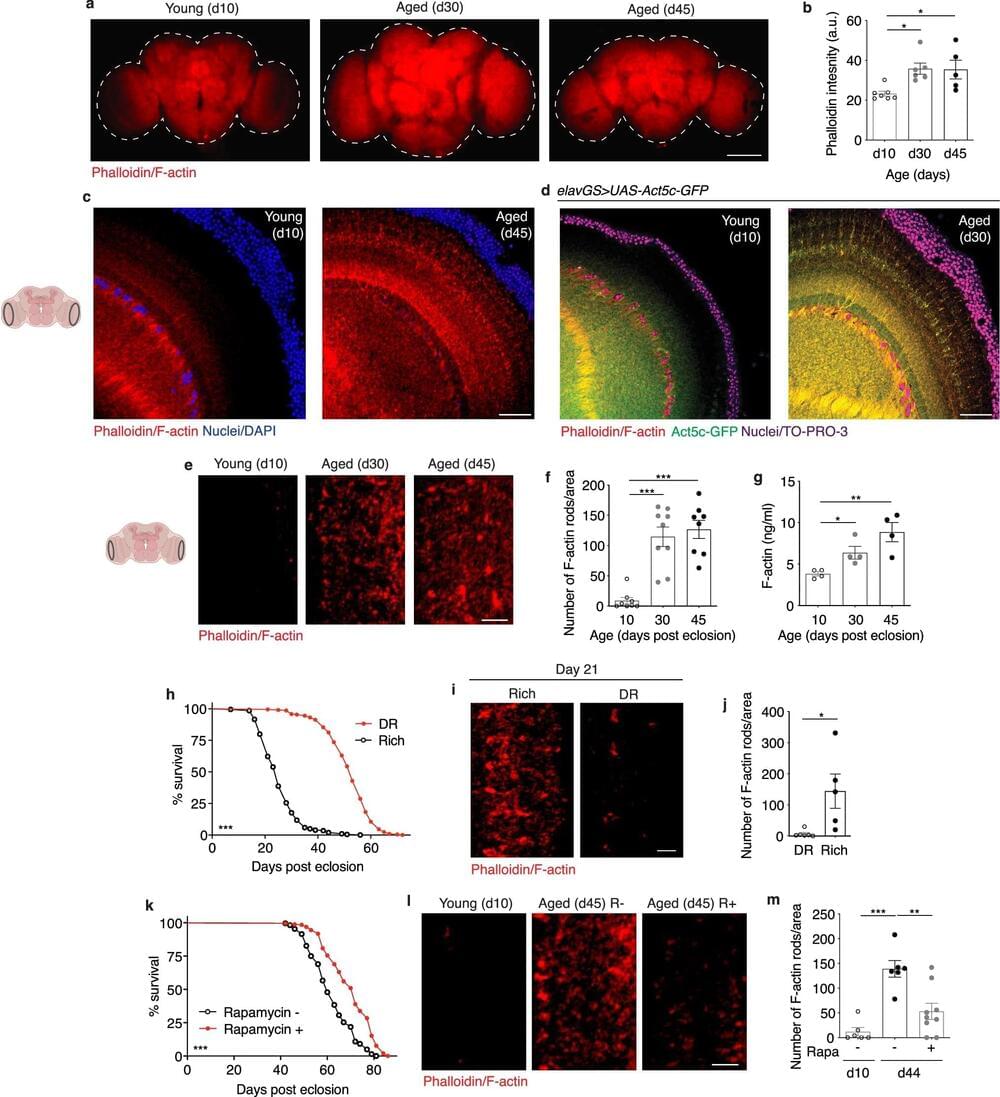
Life Biosciences is a company co-founded by the celebrity geroscientist David Sinclair and is based on his Harvard team’s research into partial cellular reprogramming. In the heated race to translate this promising technology to the clinic, Life has emerged as one of the favorites, inching closer towards clinical trials in humans. Life is counting on its proprietary reprogramming technology that uses only three out of four classic reprogramming factors and on its strong team of scientists and managers. We talked to Dr. Sharon Rosenzweig-Lipson, Life’s Chief Scientific Officer, about the company’s journey, delving deep into the technology and its future.
I’ll start by saying that Life Biosciences is one of the most exciting companies in the longevity field. You might actually become the first company to have a partial reprogramming-based therapy approved.
At Life Biosciences, we’re focused on something that matters to everyone: helping people stay healthier as they age. We’re working on what we call cellular rejuvenation technologies, basically finding ways to turn back the clock in cells and make them more youthful. I came on board as Chief Scientific Officer about a year and a half ago, but I actually got to know the company pretty well before that. I consulted for them for a year, which gave me a chance to look under the hood, see the science they were doing, and I got really excited about what I saw.