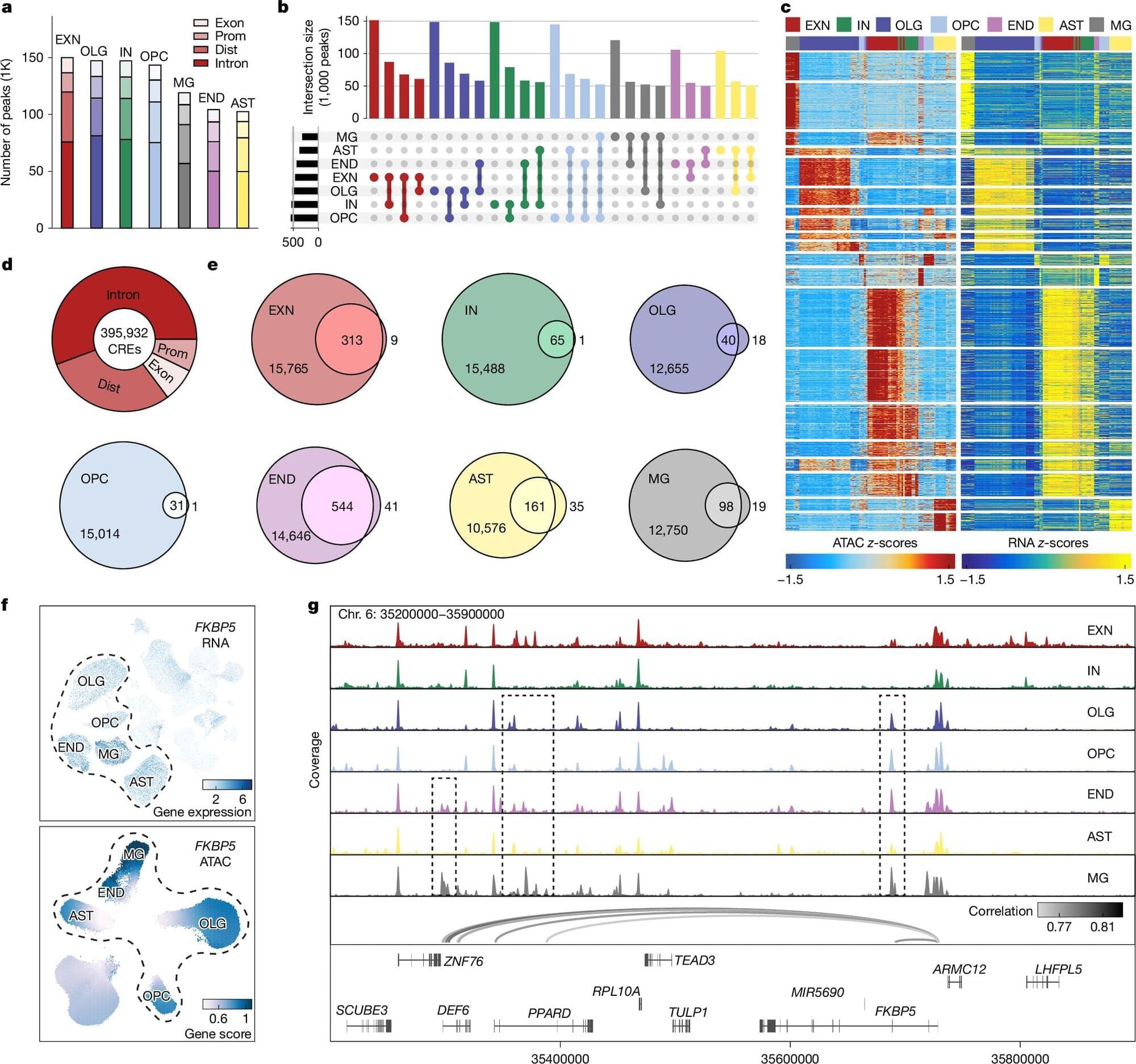
The human brain is made up of billions of interconnected cells that are constantly talking to each other. A new study published in Nature zooms in to the single-cell level to see how this cellular communication may be going wrong in brains affected by post-traumatic stress disorder (PTSD).
Until recently, researchers did not have the technology to study genetic variation within individual cells. But now that it’s available, a team led by Matthew Girgenti, Ph.D., assistant professor of psychiatry at Yale School of Medicine, has been analyzing brain cells to uncover genetic variants that might be associated with psychiatric diseases such as major depressive disorder (MDD) and PTSD.
Their latest study is one of the first to examine a major psychiatric disorder, PTSD, at the single-cell level. For years, doctors have been prescribing antidepressants to treat the condition because there are currently no drugs specifically designed for PTSD. Girgenti hopes that identifying novel molecular signatures associated with the psychiatric disease can help researchers learn how to develop new drugs or repurpose existing ones to treat it more effectively.