Genetic transcription is a data problem, and AI is on the case.


Originally published on Illumina News Center
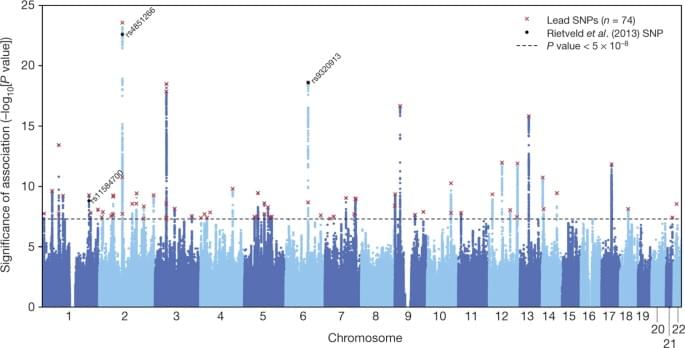
Call it archaeology by other means. Rather than sifting through tons of dirt and carefully cataloguing human artifacts, Eske Willerslev and his colleagues have used Illumina NovaSeq Systems to sequence 5,000 ancient human genomes, revealing previously unseen historical nuance. This research tour de force, which is being published this month in four papers in the journal Nature, offers a rich view of early human migrations, mating habits, and disease variants, and their impact on modern Europeans.
“We wanted to sequence this ancient DNA so we could better understand human history,” says Willerslev, who is professor and director at the Centre of Excellence in GeoGenetics at the University of Copenhagen and the Prince Philip Professor of Ecology & Evolution at Cambridge University. “These results describe where we came from and why there’s so much variation in disease risk.”
Researchers have found that treating seeds with ethylene gas increases both their growth and stress tolerance. This discovery, involving enhanced photosynthesis and carbohydrate production in plants, offers a potential breakthrough in improving crop yields and resilience against environmental stressors.
Just like any other organism, plants can get stressed. Usually, it’s conditions like heat and drought that lead to this stress, and when they’re stressed, plants might not grow as large or produce as much. This can be a problem for farmers, so many scientists have tried genetically modifying plants to be more resilient.
However plants modified for higher crop yields tend to have a lower stress tolerance because they put more energy into growth than into protection against stresses. Similarly, improving the ability of plants to survive stress often results in plants that produce less because they put more energy into protection than into growth. This conundrum makes it difficult to improve crop production.
Autism is characterized by impairments in social communication and interaction and restricted and repetitive behaviors. In this video, I discuss the neuroscience of autism along with potential factors and mechanisms involved in the development of autism.
TRANSCRIPT:
Autism, also known as autism spectrum disorder, is characterized by symptoms that include impairments in social communication and interaction and restricted and repetitive behaviors. Although the neuroscience of autism is still poorly understood, autism is considered to be a complex developmental disorder that involves atypical brain organization starting early in development.
Individuals with autism often experience a period of unusually rapid brain growth in infancy and early childhood. This accelerated brain growth is linked to an atypical pattern of connectivity between brain regions. A number of studies report that alterations in brain circuitry involved with social interaction and attention can be detected well before the symptoms of autism begin to appear. At this point, however, it’s unclear how brain overgrowth and atypical connectivity might be linked to the occurrence of autism symptoms.
Research suggests that the risk of autism is strongly influenced by genetics, yet studies consistently report that environmental factors also play a large role. Although a number of potential environmental factors have been identified, the risk factors for autism are far from definitive, and it remains unclear which factors are responsible for causing an increase in autism risk, and which are associated in a non-causal way. The risk factors that are most strongly linked to autism are associated with the prenatal or perinatal period. Thus, it’s possible they might be responsible for disruptions to typical neural development, leading to symptoms of autism months or years later. How these risk factors might interfere with neural development is still uncertain, but hypotheses have suggested potential mechanisms such as epigenetic effects, inflammation, oxidative stress, or damage caused by oxygen deficiency. More work needs to be done, however, to fully elucidate the genetic and environmental risk factors for autism, as well as the mechanisms for the development of autism symptoms.
REFERENCES:
Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhDDiscount Links: Telomere, Epigenetic Testing: https://trudiagnostic.com/?irclickid=U-s3Ii2r7x…
The various identities of cells, whether they are in the brain, heart, kidney, or any other tissue, are defined by the genes they expressed. In basic terms, the genes that are active in a cell are transcribed into RNA molecules that are then translated into proteins using tRNA molecules. In the genetic code, three base pair sequences of DNA, or codons, represent amino acids. These amino acids are moved into place by tRNA molecules, which have matching anticodons, to make proteins. There is redundancy in the genetic code as well, in which one amino acid can often be encoded by a few different codons.
Protein production varies considerably in different cells, and this is especially notable in cells that generate antibodies. These cells often have to spring into action and shift into high gear to generate many infection-fighting antibodies quickly. These antibody producers are B cells, and they often make significant metabolic adaptations when they’re needed.

The random nature of genetic mutation implies evolution is largely unpredictable. But recent research suggests this may not be entirely so, with interactions between genes playing a bigger role than expected in determining how a genome changes.
It’s known that some areas of the genome are more likely to be mutable than others, but a new study now suggests a species’ evolutionary history may play a role in making mutations more predictable too.
“The implications of this research are nothing short of revolutionary,” says University of Nottingham evolutionary biologist James McInerney.
Give people a barrier, and at some point they are bound to smash through. Chuck Yeager broke the sound barrier in 1947. Yuri Gagarin burst into orbit for the first manned spaceflight in 1961. The Human Genome Project finished cracking the genetic code in 2003. And we can add one more barrier to humanity’s trophy case: the exascale barrier.
The exascale barrier represents the challenge of achieving exascale-level computing, which has long been considered the benchmark for high performance. To reach that level, however, a computer needs to perform a quintillion calculations per second. You can think of a quintillion as a million trillion, a billion billion, or a million million millions. Whichever you choose, it’s an incomprehensibly large number of calculations.
On May 27, 2022, Frontier, a supercomputer built by the Department of Energy’s Oak Ridge National Laboratory, managed the feat. It performed 1.1 quintillion calculations per second to become the fastest computer in the world.