Near-gapless and haplotype-resolved genome assemblies of the dwarfing ‘M9’ and semi-vigorous ‘MM106’ rootstocks and a major apple cultivar ‘Fuji’ provide insights into the genetic basis of rootstock-induced dwarfing traits.
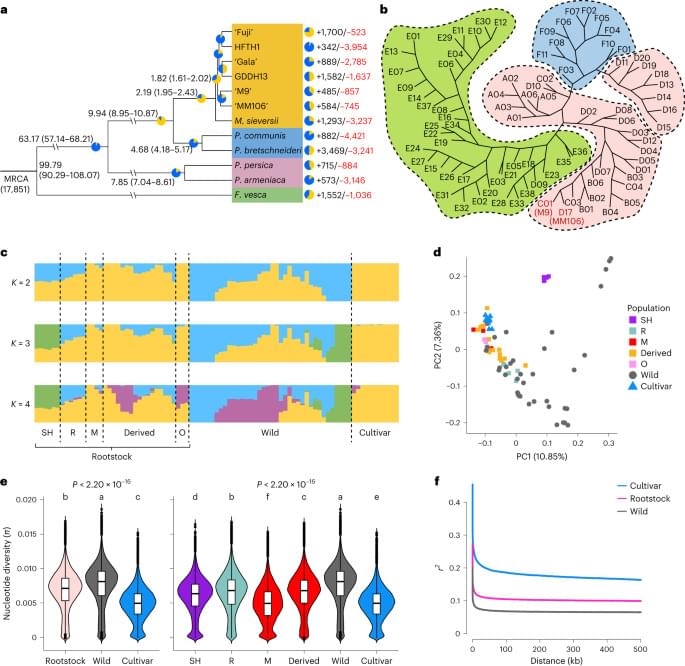

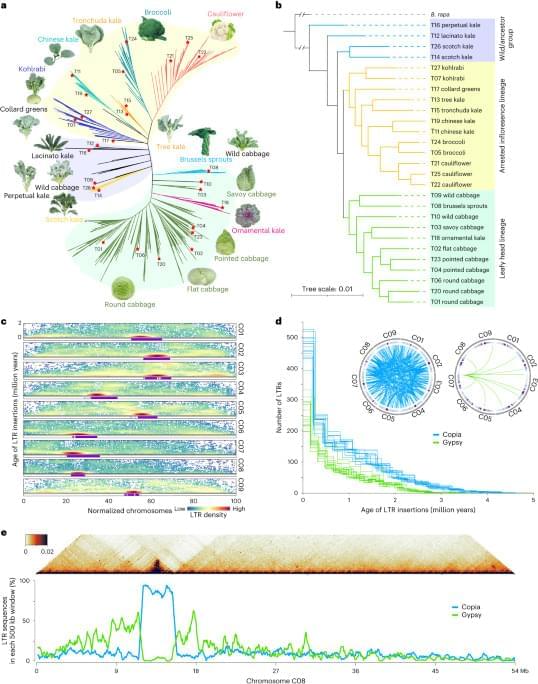
To construct a pan-genome that encompasses the full range of genetic diversity in B. ole racea, we analyzed the resequencing data of 704 globally distributed B. ole racea accessions covering all different morphotypes and their wild relatives (Supplementary Tables 1 and 2). We identified 3,792,290 SNPs and 528,850 InDels in these accessions using cabbage JZS as reference genome22. A phylogenetic tree was then constructed using SNPs, which classified the 704 accessions into the following three main groups: wild B. ole racea and kales, arrested inflorescence lineage (AIL) and leafy head lineage (LHL; Fig. 1a and Supplementary Note 2). The phylogenetic relationship revealed in our study was generally consistent with those reported previously4,5,24,25. Based on the phylogeny and morphotype diversity, we selected 22 representative accessions for de novo genome assembly (Table 1).
We assembled genome sequences of the 22 accessions by integrating long-reads (PacBio or Nanopore sequencing), optical mapping molecules (BioNano) or high-throughput chromosome conformation capture data (Hi-C) and Illumina short-reads (Methods; Supplementary Note 2 and Supplementary Tables 3–7). The total genome size of these assemblies ranged from 539.87 to 584.16 Mb with an average contig N50 of 19.18 Mb (Table 1). An average of 98% contig sequences were anchored to the nine pseudochromosomes of B. ole racea. The completeness of these genome assemblies was assessed using benchmarking universal single-copy orthologs (BUSCO), with an average of 98.70% complete score in these genomes (Supplementary Table 8).
To minimize artifacts that could arise from different gene prediction approaches, we predicted gene models of both the 22 newly assembled genomes and the five reported high-quality genomes5,21,22,23 using the same annotation pipeline (Methods). Using an integrated strategy combining ab initio, homology-based and transcriptome-assisted prediction, we obtained a range of 50,346 to 55,003 protein-coding genes with a mean BUSCO value of 97.9% in these genomes (Table 1). After gene prediction, a phylogenetic tree constructed based on single-copy orthologous genes clustered the 27 genomes into three groups, similar to the results observed in the population (Fig. 1a and b).

The cultivation of triploid genetics could be the game changer for the cannabis industry, as it promises to deliver higher THC levels, larger yields, faster growth, and seedless flowers.
The application of triploids is not a new concept in agriculture. Consuming seedless fruit generally enhances the eating experience for most people.
Consider bananas, for instance. Bananas lack seeds because the parent banana tree is triploid, even though pollination normally occurs.

Diagnosing schizophrenia as early as possible helps minimize the toll the neurological disorder takes on the body and the mind. Unfortunately the condition’s signs can be difficult to spot in the early stages.
That’s why researchers led by a team from the Indiana University School of Medicine have developed a test which offers a relatively simple and reliable way to check for current schizophrenia severity and future risk.
“Psychosis usually manifests in young adulthood – a prime period of life,” says neuroscientist Alexander Niculescu from the Indiana University School of Medicine. “Stress and drugs, including marijuana, are precipitating factors on a background of genetic vulnerability.”
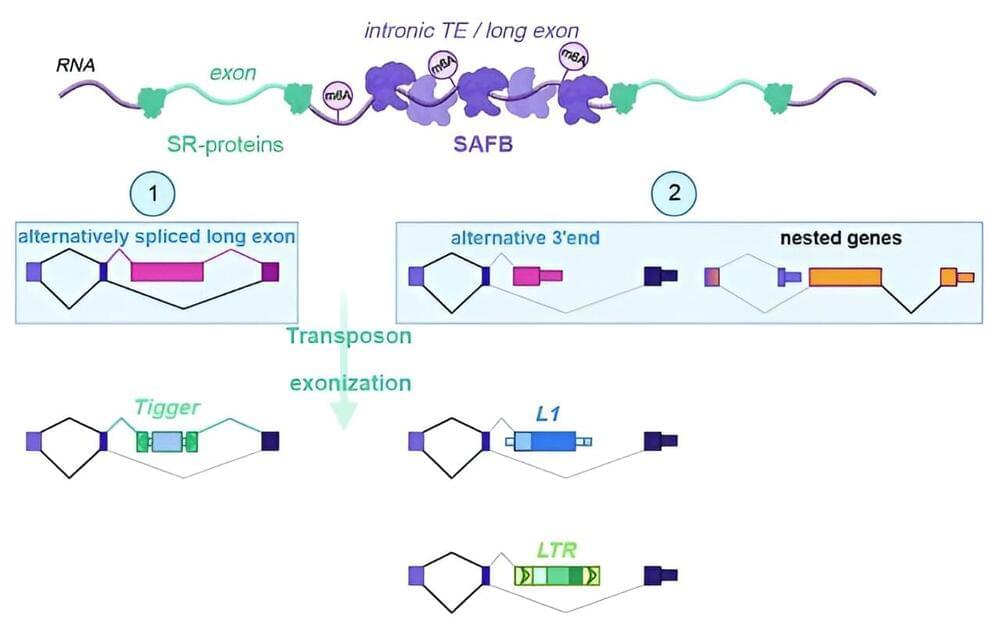
Transposable elements are mobile genetic elements that can relocate within the genome and disrupt the normal function of genes, but are at the same time a source of evolutionary diversity. The lab of Tugce Aktas at the Max Planck Institute for Molecular Genetics has identified a novel pathway that keeps the activity of transposons in somatic cells in check after they have been transcribed.
Their findings have now been published in Nature. The work is a collaboration with the labs of Zachary D. Smith at the Yale Stem Cell Center, U.S., and Franz-Josef Müller from the Universitätsklinikum Schleswig-Holstein, Germany.
Over the course of evolution, the genomes of many organisms have become cluttered with ancient genetic remnants from evolution or parts of retroviruses that inserted their genetic code millions of years ago. Nearly half of the human genome consists of these transposable elements, or transposons.
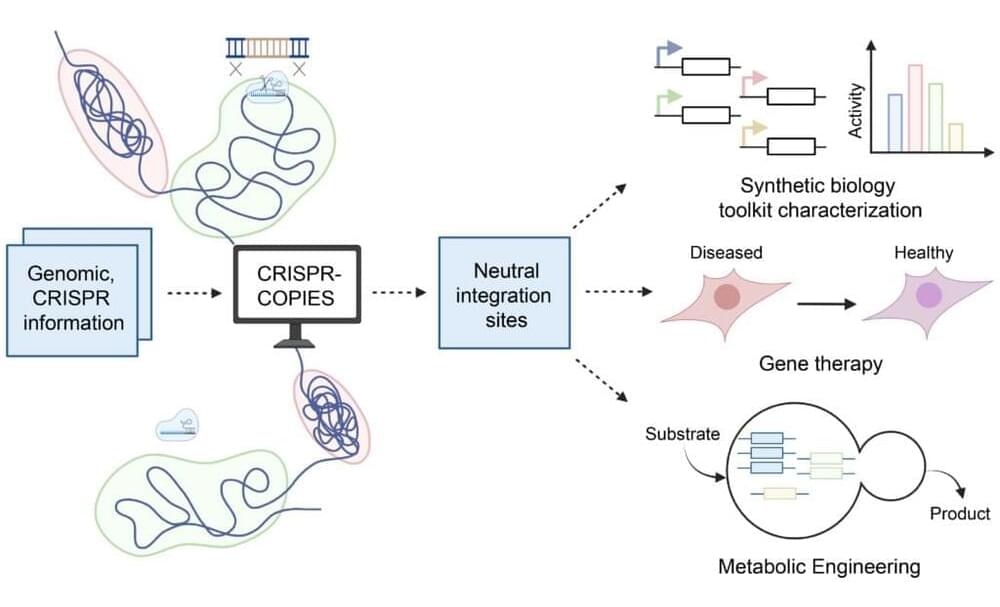
CRISPR/Cas systems have undergone tremendous advancement in the past decade. These precise genome editing tools have applications ranging from transgenic crop development to gene therapy and beyond. And with their recent development of CRISPR-COPIES, researchers at the Center for Advanced Bioenergy and Bioproducts Innovation (CABBI) are further improving CRISPR’s versatility and ease of use.
“CRISPR-COPIES is a tool that can quickly identify appropriate chromosomal integration sites for genetic engineering in any organism,” said Huimin Zhao, CABBI Conversion Theme Leader and Steven L. Miller Chair of Chemical and Biomolecular Engineering (ChBE) at the University of Illinois. “It will accelerate our work in the metabolic engineering of non-model yeasts for cost-effective production of chemicals and biofuels.”
Gene editing has revolutionized scientists’ capabilities in understanding and manipulating genetic information. This form of genetic engineering allows researchers to introduce new traits into an organism, such as resistance to pests or the ability to produce a valuable biochemical.

The authors of a recent review published in Ageing Research Reviews summarize the research on epigenetic reprogramming and its potential as a rejuvenation therapy [1].
Aging leads to changes in the epigenome. Those changes can lead to alterations in gene regulation, affecting cellular homeostasis, and can play a role in age-associated phenotypes. Epigenetic modifications, the addition or removal of chemical groups to the DNA or DNA-associated proteins, have a profound impact on gene expression, tissue functions, and identity [2].
This review’s authors believe epigenetic reprogramming to be among the most currently promising interventions to stop or delay aging, potentially even reversing it at the cellular level. They believe that epigenetics are the basis of aging; therefore, being able to impact the epigenome would allow them to address multiple Hallmarks of Aging simultaneously.
City of Hope, one of the largest cancer research and treatment organizations in the United States, treated the oldest person to be cured of a blood cancer and then achieve remission for HIV after receiving a blood stem cell transplant from a donor with a rare genetic mutation. Research published in the New England Journal of Medicine today demonstrates that older adults with blood cancers who receive reduced intensity chemotherapy before a stem cell transplant with donor cells that are resistant to HIV may be cured of HIV infection.
Paul Edmonds, 68, of Desert Springs, California, is the fifth person in the world to achieve remission for acute myelogenous leukemia and HIV after receiving stem cells with a rare genetic mutation, homozygous CCR5 Delta 32. That mutation makes people who have it resistant to acquiring HIV. Edmonds is also the person who had HIV the longest—for over 31 years—among these five patients.
Known as the “City of Hope patient” among these five patients, Edmonds received a transplant at City of Hope on Feb. 6, 2019, and is now considered to be cured of leukemia. Edmonds stopped taking antiretroviral therapies for HIV nearly three years ago and will be considered cured of HIV after he has stopped taking antiretrovirals for five years.
Summary: Researchers uncovered the mechanisms by which oxytocin (OXT) influences learning and memory in animals. Their study utilized pharmacogenetic techniques to activate specific OXT neurons within the brain, assessing the impact on cognitive functions through tasks like the Novel Object Recognition Task (NORT).
The findings reveal that activating OXTergic neurons significantly enhances long-term object recognition memory, with notable activity observed in the brain’s supramammillary nucleus (SuM) and dentate gyrus. This groundbreaking research not only deepens our understanding of oxytocin’s role beyond social bonding but also suggests its potential in developing treatments for dementia.

In the realm of scientific innovation, the past decade has seen the CRISPR/Cas systems emerge as a groundbreaking tool in genome editing, boasting applications that span from enhancing crop yields to pioneering gene therapy.
The recent advent of CRISPR-COPIES by the Center for Advanced Bioenergy and Bioproducts Innovation (CABBI) marks a significant leap forward, refining CRISPR’s flexibility and user-friendliness.
CRISPR-COPIES represents a cutting-edge solution designed to swiftly pinpoint ideal chromosomal sites for genetic modification across any species.